
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pleasssssseeee solve this question in cheeemsirty, thankss sir
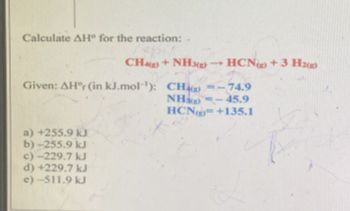
Transcribed Image Text:Calculate AH° for the reaction:-
CH()+ NH3g) -HCN) +3.H2(g)
Given: AH°r (in kJ.mol): CH()=-74.9
a) +255.9 kJ
b)-255.9 kJ
c)-229.7 kJ
d) +229.7 kJ
e)-511.9 kJ
NH)-45.9
HCN+135.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 1. For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆rH° = 181.6 kJ/mol-rxn. What is the enthalpy change to decompose 1.00 mol of HgO(s) to O2(g) and Hg(l)? 3633 kJ −90.8 kJ 90.8 kJ 363.3 kJarrow_forward10.46 Discuss the effect of temperature change on the spontaneity of the following reactions at 1 atm. (a) Al2O3(s)+2Fe(s)2Al(s)+Fe2O3(s) H = 851.5kJ; S =38.5 J/K (b) N2H4(l)N2(g)+2H2(g) H =-50.6 kJ; S= 0.3315 kJ/K (c) SO3(g)SO2(g)+12O2(g) H = 98.9 kJ; S= 0.0939 kJ/Karrow_forwardWhat determines Ssurr for a process? To calculate Ssurr at constant pressure and temperature, we use the following equation: Ssurr = H/T. Why does a minus sign appear in the equation, and why is Ssurr inversely proportional to temperature?arrow_forward
- When 7.11 g NH4NO3 is added to 100 mL water, the temperature of the calorimeter contents decreases from 22.1 C to 17.1 C. Assuming that the mixture has the same specific heat as water and a mass of 107 g, calculate the heat q. Is the dissolution of ammonium nitrate exothermic or endothermic?arrow_forwardGiven the following information at 25C, calculate G at 25C for the reaction 2A(g)+B(g)3C(g) Substance Hf(kJ/mol) S(J/molK) A(g) 191 244 B(g) 70.8 300 C(g) 197 164 a 956 kJ b 956 kJ c 346 kJ d 346 kJ e 1.03 103 kJarrow_forwardFor the reaction TiCl2(s) + Cl2(g) TiCl4(), rG = 272.8 kj/mol-txn. Using this value and other data available in Appendix L, calculate the value of fG for TiCl2(s).arrow_forward
- Would the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or remain the same if the heat capacity of the calorimeter were taken into account? Explain your answer.arrow_forward9.83 A student performing a calorimetry experiment combined 100.0 mL of 0.50 M HCl and 100.0 mL of 0.50 M NaOH in a coffee cup calorimeter. Both solutions were initially at 20.0°C, but when the two were mixed, the temperature rose to 23.2°C. (a) Suppose the experiment is repeated in the same calorimeter but this time using 200 mL of 0.50 M HCl and 200.0 mL of 0.50 M NaOH. Will the T observed he greater than, less than, or equal to that in the first experiment, and why? (b) Suppose that the experiment is repeated once again in the same calorimeter, this time using 100 mL of 1.00 M HCl and 100.0 mL of 1.00 M NaOH. Will the T observed he greater than, less than, or equal to that in the first experiment, and why?arrow_forwardCalculate rS for the following reaction at 25 C. 2 H2(g) + O2(g) 2 H2O() (a) 326.6 J/K mol-rxn (b) 139.9 J/K mol-rxn (c) 139.9 J/K mol-rxn (d) 326.6 J/K mol-rxnarrow_forward
- Another step in the metabolism of glucose, which occurs after the formation of glucose6-phosphate, is the conversion of fructose6-phosphate to fructose1,6-bisphosphate(bis meanstwo): Fructose6-phosphate(aq) + H2PO4(aq) fructose l,6-bisphosphate(aq) + H2O() + H+(aq) (a) This reaction has a Gibbs free energy change of +16.7 kJ/mol of fructose6-phosphate. Is it endergonic or exergonic? (b) Write the equation for the formation of 1 mol ADP fromATR for which rG = 30.5 kJ/mol. (c) Couple these two reactions to get an exergonic process;write its overall chemical equation, and calculate theGibbs free energy change.arrow_forwardGiven that H f for HF(aq) is -320.1 kJ/mol and S for HF(aq) is 88.7 J/mol K, find Ka for HF at 25C.arrow_forwardSuppose you have an endothermic reaction with H = + 15 kJ and a S of 150 J/K. Calculate G and Keq at 10, 100, and 1000 K.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
