
Concept explainers
(a)
Interpretation:
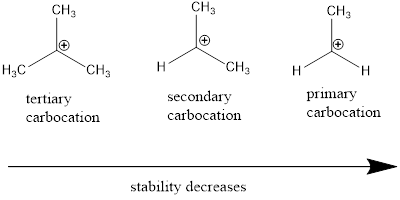
From the given compounds, the compound with high regioselective ability towards addition of
Concept introduction:
Addition Reaction: It is defined as
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
(b)
Interpretation:
From the given compounds, the compound with high regioselective ability towards addition of
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Give a resonance structure for the following compound and explain why it is more stable than an aliphatic diazonium saltarrow_forward(a)Which compound/s will produce an orange precipitate upon reaction with 2,4-DNPH? (b)Which compound/s will test positive towards Baeyer’s test? (c)Which compound/s will produce a brick-red precipitate upon reaction with Fehling’s reagent (CuSO4, tartrate, NaOH)? (d)Which compound/s will test positive towards the Iodoform test?arrow_forwardTreatment of 1,3,6-cyclononatriene (Compound 1), or its dimethyl derivative (Compound 2), with potassium amide (KNH₂) in liquid ammonia results in the formation of anion 1a or 2a, respectively (J. Am. Chem. Soc. 1973, 95, 3437-3438): 9 15.85a 2 6 3 4 5 Compound 1 (R=H) Compound 2 (R=CH3) * You answer is incorrect. KNH₂ KNH₂ 1a (anion) 2a (anion) Of the following, which is NOT one of the four resonance structures of 1a?arrow_forward
- 11) In electrophilic aromatic substitution reactions the nitro group is: A) a m-director since it destabilizes the meta sigma complex more than the ortho, para. B) a m-director since it destabilizes the meta sigma complex less than the ortho, para. C) an o,p-director since it stabilizes the ortho, para sigma complex more than the meta. D) an o,p-director since it stabilizes the ortho, para sigma complex less than the meta. E) none of the above.arrow_forwardIdentify the compound(s) that will readily undergo McLafferty rearrangement during a mass spectral analysis. NH₂ O Only I O Only II O Only III O Only I and II Only I and III O Only II and III O All three IIIarrow_forwardDraw the structure of the compound that produced the spectra below. The infrared spectrum has strong bands at 1720 and 1738 cm-1.arrow_forward
- Treatment of the bicyclic chloride A with lithium in tetrahydrofuran (THF) gave the monocyclic product B, C,H,Li. In deuterated THF, B exhibited a single proton NMR signal at 6.72 ppm and a single 13 C-NMR signal at about 110 ppm. Treatment of B with tetraethylammonium chloride gave a white solid in which N(C2H5)4 replaces Li. Draw a structure for the lithium derivative B. A Li/THF -CI CgHgLi (C2H5)4N CI C9H9N(C2H5)4 B Draw cations and anions in separate sketchers. ⚫ Separate structures with + signs from the drop-down menu.arrow_forwardA and B, isomers of molecular formula C3H5Cl3, are formed by the radical chlorination of a dihalide C of molecular formula C3H6Cl2. a.Identify the structures of A and B from the following 1H NMR data: Compound A: singlet at 2.23 and singlet at 4.04 ppm Compound B: doublet at 1.69, multiplet at 4.34, and doublet at 5.85 ppm b.What is the structure of C?arrow_forward1. An aliphatic ketone absorbs at 1,715 cm-1. What is the frequency of this vibration in hertz, which is cycles per second or just per second, reciprocal seconds? 2. What is the energy equivalent of this stretching vibration in kcal/mole? 3. Why does 3,4-diethyl-3-hexene not have a carbon to carbon double bond stretching absorption band? 4. Why does a carbon to oxygen double bond absorption band have a greater intensity than a carbon to carbon double bond absorption band? 5. Using only IR, explain in detail how one could most easily differentiate between oct-1-ene and oct-1-yne if all carbon to carbon bonds are ignored. 6. Using only IR, explain in detail how one could most easily differentiate between butan-1-ol and butanoic acid.arrow_forward
- If compound U shows the same reaction time as carbon tetrachloride under identical GC/MS conditions, compound U:arrow_forwardAnswer the following question pertaining to the reaction of maleic anhydride and 1,3-butadiene: Why does 1,3-butadiene react more rapidly with maleic anhydride than with another molecule of itself?arrow_forwardFor each of the following absorption bands, indicate whether you would expect to see that band in the IR spectrum of 3,5-dimethyl-N-phenylbenzamide: 3,5-dimethyl-N-phenylbenzamide A: One sharp absorption band at ~1775 cm 1 [ Select] B: One broad absorption band at ~3400 cm1 [Select ] C: Two weak absorption bands at ~2700 cm-1 and ~2800 cm 1 [ Select] D: One sharp absorption band at ~1675 cm1 [Select ] > > >arrow_forward
