
Interpretation:
The all enol tautomers for each of the
Concept Introduction:
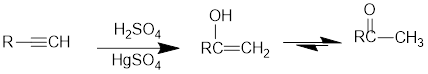
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and an
If a carbonyl group is bonded to two alkyl groups, it is called as a ketone. The enol formed in the acid catalysed addition of water will be easily converted into a ketone.
Conversion of terminal
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Label each pair of compounds as keto–enol tautomers or constitutional isomers, but not tautomers.arrow_forwardPlease draw substitution and elimination major productsarrow_forwardThe major product of the given reaction has the molecular formula CH03. Draw its structure in the most stable tautomeric form. Select Draw Rings More Erase H 1. NaOCH,CH3 2. H3O* CH;CH2OHarrow_forward
- Draw the keto- tautomer for the following enolsarrow_forwardThe major product of the given reaction has the molecular formula C10H16O3. Draw its structure in the most stable tautomeric form.arrow_forwardProvide a complete step-by-step mechanism for the following reaction. ОН Conc. H2SO4 heatarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





