
Concept explainers
(a)
Interpretation:
For the given set of species the available
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Cation: The positively charged chemical species is referred as cation.
(b)
Interpretation:
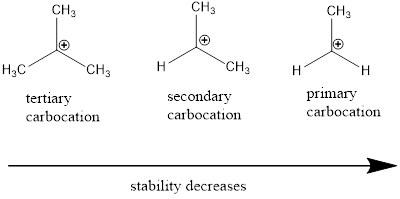
From the given carbocation the most stable one has to be identified.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Cation: The positively charged chemical species is referred as cation.
Carbocation stability order:
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Complete the sentence. Hyperconjugation describes how a carbocation is stabilized when the electrons of ____ donate electron density into____. A. a pi bond / an eclipsed C:H bond B. radical / the adjacent pi bond C. a double bond / the positive carbon D. a sigma bond / an empty p orbitalarrow_forwardWhat are the bond-line formulas for the following compounds? a. 6-butyl-4,7-diethylundecaneb. 1-bromo-3-ethyl-5-methylcyclohexanec. trans-1,3-dimethylcyclopentane d. 1-bromopropane (the most stable Newman projection)e. trans-1,2-dibromocyclohexane (the most stable chair conformation)arrow_forwardConsider 1– Bromo – 2– methylbutane. A. Looking down at the C1:C2 bond draw the Newman projection with the lowest energy B. looking down at the C1:C2 bond draw the Newman projection highest energyarrow_forward
- A terminal alkyne (RC=CH) is exposed to excess HBr. What rule should be followed to determine the placement of the halogen atoms in the product? A. Markovnikov rule O B. Hofmann's rule O C. Zaitzev's rule D. Anti-Markovnikov rulearrow_forwardWhich of the structures is NOT a resonance structure of the following carbocation? A. OB. OC. O D. ∞arrow_forward41.Substitution reactions that can have methy and hydride shifts are? A B SN2 SN1 I Br H 42. Arrange the compounds in increasing order of carbocation stability? Go III III >I C SN1 & SN2 identical mis II. III< C IIarrow_forward9. Which of the following alkenes has a Z configuration?arrow_forwardWhich of the following does not contribute to the stability of benzene?a. overlapping pi electrons above and below the ring's plane b. Huckel rule c. conjugation d. nonearrow_forwardWhich of the following concepts explains why a tertiary carbocation is more stable than a primary carbocation? a. Hyperconjugation b. Resonance c. Electronegativity T d. he octet rulearrow_forward4. Carbocations can undergo rearrangements to form more stable carbocations. A fourmembered ring can expand to a five-membered ring and a five-membered ring can expand to a six-membered ring, but a six-membered ring will not expand to a seven-membered ring. Provide an explanation for why the expansion of four- and five-membered rings is favorable, but the expansion of six-membered rings is not. O ** Carrow_forward2. In aqueous solution d-glucose (or dextrose) exists in equilibrium between a linear and cyclic form shown below. A. Mark all the stereocenters in the linear form of the molecule. Number them 1,2,3, etc. B. Draw out the reaction that converts the linear form to the 6-membered ring using the arrow formalism. C. Identify the stereocenters in the 6-member ring that correspond to the stereocenters in the linear form marking them as 1,2,3, etc. The reaction also created a new stereocenter. Mark it as H -OH HO--H H--OH нон Ho но HO HO H он H-OH H но.arrow_forward1 i. What is Resonance Theory? Sate five conclusions that can be drawn from the theory. ii. State the two main experiments that were used to establish the extra stability of the benzene molecule. iii. What are the factors that confer Aromaticity to an organic molecule? iv. State the effects of substituents on a benzene derivative towards further aromatic substitution. V. Based on the above suggest the various types of substituents that can be attached to Benzene.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

