
(a)
Interpretation:
The product formed when cis- and
Concept introduction:
Addition of hydrogen halides:
In the addition of hydrogen halides over
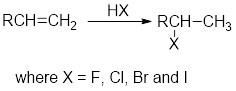
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Chiral or Asymmetric carbon: Carbon bonded with four different substituents with it is termed as chiral or asymmetric carbon.
(b)
Interpretation:
The product formed when cis- and
Concept introduction:
Addition of
Catalytic hydrogenation in presence of
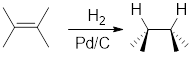
For cyclic reactants, the addition of
(c)
Interpretation:
The product formed when cis- and
Concept introduction:
Acid Catalyzed addition of water: When water is added to alkene in the presence of an acid, the product formed will be an alcohol.
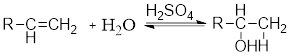
The electrophile
Nucleophile: It donates pair of electrons to positively charged chemical substrate resulting in the formation of
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Draw the organic products formed when cyclopentene is treated with each reagent. With some reagents, no reaction occurs. a.H2 + Pd-C b.H2 + Lindlar catalyst c.Na, NH3 d.CH3CO3H e.[1] CH3CO3H; [2] H2O, HO− f.[1]OsO4 + NMO; [2] NaHSO3, H2O g.KMnO4, H2O, HO− h.[1] LiAlH4; [2] H2O i. [1] O3; [2] CH3SCH3 j.(CH3)3COOH, Ti[OCH(CH3)2]4, (−)-DET k.mCPBA l.Product in (k); then [1] LiAlH4; [2] H2Oarrow_forwardDraw the organic products formed when 4-octyne is treated with each reagent. a. H2 (excess) + Pd-C b. H2 + Lindlar catalyst c. Na, NH, d. [1] Ogi (2] H,0arrow_forwardChemical Reactions: Give the product/s of each chemical reaction 1. Reaction of this alkene to the different reagents written above and below the arrows. 7 Cl₂ HBr 1. Os04 2. HIOA NBS H2O, DMSO H₂ Ptarrow_forward
- a. The following compounds have the same molecular formula as benzene. How many monobrominated products could each form? 1. HC‚CC‚CCH2CH3 2. CH2“CHC‚CCH“CH2 b. How many dibrominated products could each of the preceding compounds form? (Do not include stereoisomers.) c. How many dibrominated products could each of the compounds form if stereoisomers are included?arrow_forwardWhat substitution products are obtained when each of the following compounds is added to a solution of sodium acetate in acetic acid? a. 2-chloro-2-methyl-3-hexene b. 3-bromo-1-methylcyclohexenearrow_forwardWhat reagent (reactant) could be used to produce the product above from 1-butene? A. HBr B. Br2 C. OBR D. more than one of the above is correctarrow_forward
- Which stereoisomer would be produced from the reaction of trans-2-butene with OsO4 followed by H2O2? A. 2S,3S- diol and 2R, 3R-diol B. 2S,3R-diol and 2R,3S-diol C. 2S,3S-diol only D. 2R, 3R-diol only E. 2S,3R-diol onlyarrow_forward7) You want to synthesize 3-methyl-2-pentene from 2-chloro-3-methylpentane. Which reagent would you use? a. HCI, heat b. NH:(aq), 25°C c. CH:CO2NA, CH:CO2H, heat d. CH3CH2ONA, CH3CH2OH, heat e. CН:CH2ОН, heatarrow_forwardDetermine the product(s) formed in the following reactions. A. B. QH C=CH-CH₂ "OCH3 -C=C-CH3 CH3OH TSOH -CH₂ CH₂ OH -CH=C-CH₂ H₂O H₂SO4 OCH3 CH₂CH₂ OCH3 OCH3arrow_forward
- Draw the principal neutral organic product formed by the reaction of 2.3-dimethyl-1,3-butadiene with the given reagents. a. Excess H2 and Platinum catalyst b. 1 mole HCl (product of 1,2-addition) c. 1 mole HCl (product of 1,4-addition)arrow_forwardDraw the products formed when a-D-gulose is treated with each reagent.a. CH3I, Ag2Ob. CH3OH, HClc. Ac2O, pyridined. The product in (a), then H3O+e. The product in (b), then Ac2O, pyridinef. The product in (d), then C6H5CH2Cl, Ag2Oarrow_forwardExplain the characteristics of electrophilic addition of HX to alkenes ?arrow_forward
