
Concept explainers
(a)
Interpretation:
The given compound should be prepared using
Concept Introduction:
Addition of
Catalytic hydrogenation in presence of
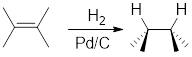
For cyclic reactants, the addition of
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
Enantiomers are given configuration as R or S based on the atoms bonded with chiral (carbon bonded with four different substituents) carbon.
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
(b)
Interpretation:
The given compound should be prepared using alkene and other reagents.
Concept Introduction:
Addition of
Catalytic hydrogenation in presence of
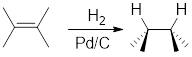
For cyclic reactants, the addition of
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
Enantiomers are given configuration as R or S based on the atoms bonded with chiral (carbon bonded with four different substituents) carbon.
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
(c)
Interpretation:
The given compound should be prepared using alkene and other reagents.
Concept Introduction:
Addition of hydrogen halides:
In the addition of hydrogen halides over alkenes, first the
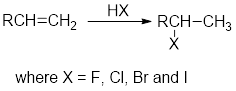
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Nucleophile: It donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Using your reaction roadmap as a guide, show how to convert cyclohexane into hexanedial. Show all reagents and all molecules synthesized along the way.arrow_forwardHow could the following compounds be prepared using an alkene as one of the starting materials?arrow_forwardPrepare the following compounds starting with cyclopentene and any other reagents.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
