
Concept explainers
Interpretation:
The compound which will be more hydrated should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:
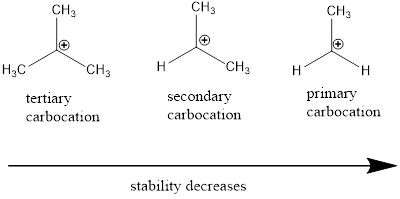
Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Rank the following in order of decreasing solubility in benzene. gallic acid ethyl benzene resorcinolarrow_forwardDraw the pyrrole that would form in each of the following reactions. a) b) COOEt NH3 Ph cat HCI, heat NH2 cat HCI, heat c) 2 equiv NH2arrow_forwardGive an acceptable name for each compoundarrow_forward
- Rank the following compounds in order of increasing boiling point (Lowest bp Highest bp) II он он IIIarrow_forwardDraw reaction of an aldehyde or a ketone with excess alcohol forms first a hemiacetal and then an acetal.arrow_forward3)What solvents could be added to increase and decrease the polarity of ethyl acetate?arrow_forward
- Rank the following compounds in order of increasing boiling point. a. Он Он Он b. но Онarrow_forwardArrange the compounds by their solubility in water. Most soluble in water -OCH3 H-C -OCH₂CH3 CH₂CH₂ -OCH3 CH₂CH₂ -OCH₂CH3 CH₂CH₂ -OCH₂CH₂CH₂ Least soluble in water įarrow_forwardWhat two functional groups react to form the following? a. A hemiacetal b. An acetal c. A ketal d. A hemiketalarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



