
(a)
Interpretation:
The major product for reaction between
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Reduction Reaction: It is just opposite of oxidation reaction which involves removal of oxygen atoms or addition of hydrogen atoms and addition of electrons.
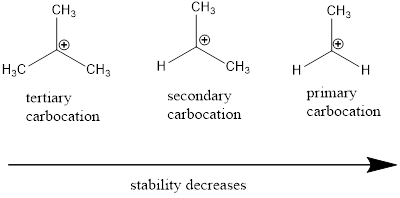
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(b)
Interpretation:
The major product for reaction between
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Reduction Reaction: It is just opposite of oxidation reaction which involves removal of oxygen atoms or addition of hydrogen atoms and addition of electrons.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

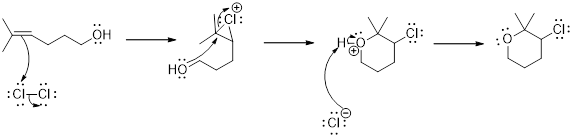
(c)
Interpretation:
The major product for reaction between
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Addition of halogen to an
(d)
Interpretation:
The major product for reaction between
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Select the best reagent expected to convert 3-heptyne to cis-3-heptene. A. NaNH2, NH3 B. Na, NH3 C. H2, Lindlar’s catalyst D. Both A and C E. Both B and Carrow_forwardA. REACTIONS OF ALKENES- provide the product(s) or reagents that are needed to accomplish the following transformations CH3 1. BH 3, THF 2. H₂O₂, NaOH, H₂Oarrow_forwardcis-Cyclohexane-1,2-diol can be synthesized from cyclohexene by using which reagent? a.O3 b.OsO4 c.H2SO4 d.mCPBAarrow_forward
- 1. Write the structure of the major organic product for reactions a, b and e? H₂C b. H₂c- CH, CH₂ Cold KMnO, OH A KMnO, H c. CH3CH₂-CH=CH-CH₂CH3 + H₂SO4 →arrow_forwardDraw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed. 1. HCl. 4.Br2 in CH2Cl2 7. H2O + H2SO4 2. BH3/THF, followed by HO-, H2O2, H2O 5. Br2 + H2O 8. CH3OH + H2SO4 3. a peroxyacid 6. H2 + Pd/Carrow_forwardWhat reagent (reactant) could be used to produce the product above from 1-butene? A. HBr B. Br2 C. OBR D. more than one of the above is correctarrow_forward
- Which stereoisomer would be produced from the reaction of trans-2-butene with OsO4 followed by H2O2? A. 2S,3S- diol and 2R, 3R-diol B. 2S,3R-diol and 2R,3S-diol C. 2S,3S-diol only D. 2R, 3R-diol only E. 2S,3R-diol onlyarrow_forward1) Which of the following the alkyl halides reacts the fastest in an SN2 reaction? * A. 1-Chloropropane B. 1-Bromopropane C. 1-Fluoropropane D. 1-Iodopropane E. Alkyl halides do not undergo SN2 reactions 2) The IUPAC name of the expected Markovnikov addition product of HI to 2-methyl-2-butene is __________.arrow_forwardProvide the reagents necessary to carry out the following transformations. Some of these may be multiple steps syntheses and may involve the use of other organic molecules. a. b. CH₂ OH Ph OHarrow_forward
- Chemical Reactions: Give the product/s of each chemical reaction 1. Reaction of this alkene to the different reagents written above and below the arrows. 7 Cl₂ HBr 1. Os04 2. HIOA NBS H2O, DMSO H₂ Ptarrow_forwardDraw the principal neutral organic product formed by the reaction of 2.3-dimethyl-1,3-butadiene with the given reagents. a. Excess H2 and Platinum catalyst b. 1 mole HCl (product of 1,2-addition) c. 1 mole HCl (product of 1,4-addition)arrow_forward3. ( Predict the organic product(s) of the reaction of 2-butene with each reagent. a. H₂O (H₂SO4) b. Cl₂ c. Br₂ in H₂Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





