
(a)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as
In addition reaction of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but differ in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation: it is carbon ion that bears a positive charge on it.
Leaving group: it is a fragment that leaves from a substrate with a pair of electrons via
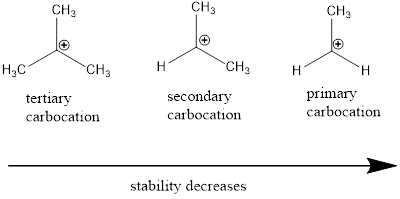
Carbocation stability order:
(b)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation stability order:

(c)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation stability order:

(d)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation stability order:

(e)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation stability order:

(f)
Interpretation:
The major product obtained after reaction of given alkene with
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the more substitution position of carbon-carbon double bond.
Carbocation stability order:

Trending nowThis is a popular solution!

Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- If the bromine group is replaced with -H. Will the yield of the product increase or decrease? Explain your reasonarrow_forwardWhat is the major organic product obtained from the following reaction? HBr Br Br Br 1 3 O 1 O 2 O 3 O 4arrow_forwardWhich of the following will reduce KMnO4 and produce a brown precipitate? 2,2-dimethylpentane Benzene Isopropylcyclohexene Hexanearrow_forward
- For the following reactions, explain which product(s) will form and why. Cro; H2SO4 PCC Cro3 HO H2SO4arrow_forwardThe major product formed in the following reaction is Br Br NaOMe Et₂Oarrow_forwardWhat is the major organic product obtained from the following reaction? Bi2 hy Br Br Br Br 1 3 4 O 1 O 2 O 3 O 4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





