
Concept explainers
(a)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:
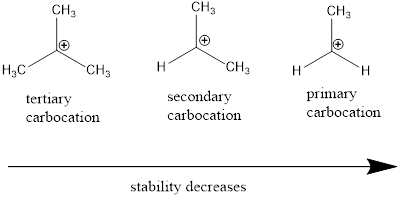
Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
(b)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:

Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
(c)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:

Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Draw the alkene that would react with the reagent given to account for the product formed. ? + HCI CH3 CH3CCH3 CI • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. #[ ] در ChemDoodlearrow_forwardRank the alkenes below from most stable to least stable.arrow_forwardWhat is the more stable carbocation. Explain itarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





