
(a)
Interpretation:
The product for given reaction should be determined.
Concept Introduction:
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a
Conversion of terminal
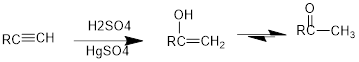
(b)
Interpretation:
The product for given reaction should be determined.
Concept Introduction:
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a ketone. The enol formed in the acid catalysed addition of water will be easily converted into a ketone.
Conversion of terminal alkynes into enol: If we want to convert terminal alkyne into an enol, the presence of mercuric ion as a catalyst should be needed and the catalyst will increase the rate of the reaction.
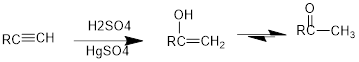
(c)
Interpretation:
The product for given reaction should be determined.
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagent is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
(d)
Interpretation:
The product for given reaction should be determined.
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for deprotonation and one of the common reagents is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





