
Concept explainers
Interpretation:
The given questions under given set of conditions should be answered.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
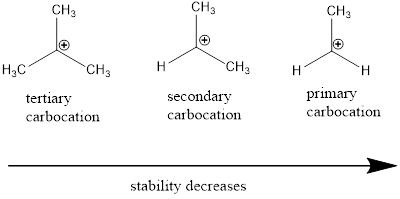
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. HO + + X S & Click and drag to start drawing a structure.arrow_forwardDraw all resonance structures for the carbocation formed by ortho attack of the electrophile +NO2 on attached starting material. Label any resonance structures that are especially stable or unstablearrow_forwardThis question has multiple parts. Work all the parts to get the most points. For the reaction below identify the reactant electrophile and the nudeophile. HO :OHarrow_forward
- What is the best electrophile to use to to make 5,5-dimethyl-2-hexyne through E2 reactions? O A. 3,4-dibromo-2,2-dimethylhexane O B. 3,5-dibromo-2,2-dimethylhexane C. 4,4-dibromo-2,2-dimethylhexane D. 4,5-dibromo-2,2-dimethylhexanearrow_forward1. What is the function of CH»Ch in the bromination reactions? Why can it fulfil this role? 2. In not more than three (3) sentences, explain why terminal alkynes are acidic. 3. What impurities are removed when acetylene gas is made to pass through an acidified solution of CuSO:? 4. Explain the difference in the rate of free radical bromination reactions of toluene and cyclohexane. 5. Give the reagent er chemical cempounde, Previde only Ehe reasents nu Would differentiate tefelarrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. NH2 + Br. + X 5 Click and drag to start drawing a structure.arrow_forward
- Polar aprotic solvents enhance the rate of an SN2 reaction by stabilizing the cations and the anions. changing the polarizibility of the nucleophile. raising the energy of the nucleophile. O lowering the energy of the nucleophile. Save for Laterarrow_forward20. a. Label the reactive features, highlight the most reactive one, then highlight what it needs. Also, state if the reaction will start to create a carbocation, carbon radical, or carbanion, or will cause loss of aromatic character. If a carbocation, carbon radical, or carbanion starts to develop, label where that will occur. HN- CH3 with 2-chloro-3-methylbutane + AICI, b. Use mechanism arrows to illustrate the reaction that occurs. c. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance-stabilized intermediate. d. Continue labeling and diagramming the reaction until you find the major stable product(s). e. Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forwardWhen HCI, HBr, and HI were used as HX in the next reaction, the rates of all reactions were almost the same. below Answer the question. OH HX CH3 CH3 H2O (1) Show the ions that act as each nucleophile in order from the one that is considered to have the highest nucleophilic reactivity. (2) Explain the reason why you thought that the order was (1). (3) Explain why the reaction rates were almost the same regardless of which HX was used. (4) Nax was used instead of HX, but the reaction did not proceed. Explain the reason for this.arrow_forward
- 9) Suppose I want to perform a simple substitution reaction, replacing the OH with a Cl in the following compound. Which of the following reagents works best? Explain (and that means explain why the wrong answers are wrong as well as why the right answer is right). Option A: Addition of HBr Option B: Addition of SOC12/pyridine Option C: Addition of SOCl2 only OH ? J1arrow_forward4. ) Rank the nucleophiles from weakest to strongest. (1 = weakest, 3 = strongest) %3D HO,arrow_forwardI need to know the products of this reaction and the mechanism of the reaction with arrows because I cannot understand the electron movement. Also which is the nucleophile and which is the electrophile?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
