
Concept explainers
(a)
Interpretation: The reasonable mechanism for product B in reaction below should be drawn.
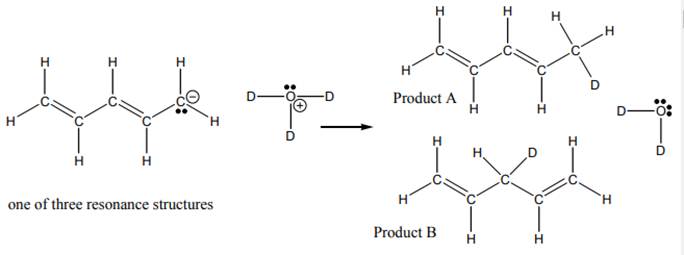
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(b)
Interpretation: The ratio
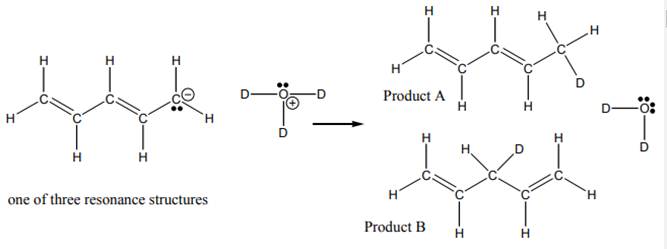
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Show work..don't give Ai generated and copy the answer anywhere.arrow_forwardthis is an inorganic chemistry question please answer accordindly!! its just one question with parts till (n) JUST ONE QUESTION with its parts spread out in the form of different images attached 2 IMAGES ATTACHED PLEASE SEE BOTH, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, graphs or diagrams, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details as needed EACH PART CLEARLY please or let another expert handle it thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- this is an inorganic chemistry question please answer accordindly!! its just one question with parts till (g) JUST ONE QUESTION with its parts spread out in the form of different images attached 2 IMAGES ATTACHED PLEASE SEE ALL, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardThe complex anion in Ba₂[Cr(CN)6] is a tetragonally distorted octahedral complex (Dan). Baz[Cr(CN)6] is paramagnetic at room temperature with S = 1. Assume that the complex is a low-spin complex. a) Identify if the [Cr(CN)6] anionic complex has 4 long and 2 short bonds (left side of figure) or if the complex has 4 short and 2 long bonds (right side of figure) with respect to Oh symmetry. Use crystal field theory to answer this question. Explain/rationalize your decision. Can the provided information decide on the order of orbital energies? Dah Tetragonal Distortion ய Dab z-compression z-elongation x and y elongation O symmetry x and y compression E eg d² dx²-y² t2g dxy dxz dyz Question 4 a) continued: Provide your explanations in the space below. b) At low temperatures Ba₂[Cr(CN)6] is ferromagnetically ordered with a phase transition to a paramagnetic phase at Tc = 150K. Sketch the magnetic susceptibility vs. temperature in the diagram below. Indicate Tc as well as the paramagnetic and…arrow_forwarda) Draw the octahedral mer-[FeCl3(CN)3] complex and determine its point group. Use proper wedges and dashes in order to illustrate 3 dimensional details. Use the point group to determine if the complex has a resulting net dipole moment and describe its allowed direction with respect to its symmetry elements (if applicable). ード M 4- b) Substitute one chlorido ligand in mer-[FeCl3(CN)3] 4 with one fluorido ligand. Determine all possible isomers and their corresponding point groups. Use the point groups to determine if the complexes have resulting net dipole moments and describe their allowed direction with respect to its symmetry elements (if applicable). The number of complex sketches below is not necessarily indicative of the number of isomers. 4- 4- ☐☐☐ c) Substitute two chlorido ligands in mer-[FeCl3 (CN)3] 4 with two fluorido ligands. Determine all possible isomers and their corresponding point groups.. Use the point groups to determine if the complexes have resulting net dipole…arrow_forward
- Draw the trigonal prismatic MH6 molecular compound, where M is a 3d transition metal. a) Draw the trigonal prismatic MH6 molecular compound and determine its point group. b) i. What is the symmetry species for the 4s orbital on the central metal? ii. What is the symmetry species for the 3dx²-y² orbital on the central metal? Note: The z-axis is the principal axis. iii. Suggest a crystal field energy diagram for a d² electron configuration in a trigonal prismatic coordination environment. Label the metal d-orbital with their corresponding symmetry species label. Use the appropriate character table in the resource section.arrow_forwardPlease correct answer and don't used hand raiting don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





