
Concept explainers
Interpretation:The most acidic hydrogen in pair of molecules given below should be circled. Also, molecule that haslower value of
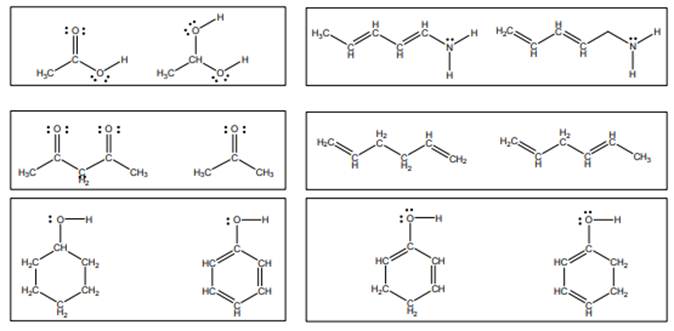
Concept introduction:Lewis structure is representation of molecule in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of the electrons in the molecule.
When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- For each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forwardComplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forwardComplete each Lewis structure, draw all important resonance structures, predict a value for thebond angles requested, and explain your reasoning. a. Nitrous acid (HNO2)HONOHON=ONO= b. Enolate ion (C2H3O) HC1C2=HC2C1=arrow_forward
- A) for each compound show its conjugate base. lone pairs have been left out. B) rank the conjugate base in the order you would predict, from most to least stable. C) rank the original compounds in order, from strongest to weakest acid.arrow_forwardFor the following molecules, identify the acidic hydrogens from among those that are bolded. For each acidic hydrogen that you identify, draw the conjugate base that results from a base removing that acidic hydrogen. For molecules with more than one acidic hydrogen, draw a separate conjugate base for each one.arrow_forwardIn each pair of compounds below decide which one is more acidic.arrow_forward
- Rank the following compounds from most basic to least basic. Briefly explain your reasoning which may include the use of pKa values. In case you can't read them those are all negative 1 charges in the small circles. NH A Barrow_forward5. Rank each of the five compounds by relative basicity. Use a "1" for the strongest base, a "2" for the 2nd strongest, all the way to a "5" for the weakest base. NH2 NH2 NH2 MeO NH2 NH2 O2Narrow_forwardBr NH₂ OH NH₂ CH3 A/RO/exception Circle the more acidic compound and determine the effect responsible for such observation using ARIO methods, or if its an exception to the rule. Please explain thanks.arrow_forward
- a) Draw the conjugate base of the compound shown. Include all resonance structures. b) The pKa of phenol is 10. Does the compound shown have a pKa greater or less than 10? Fully explain your answer. (Hint: “It’s more acidic” or “it’s less acidic” is not a full explanation)arrow_forwardExplain why the C-H, bond is much more acidic than the C-H, bond in pentan-2-one. Select the single best answer. HH. perfar 0H, is more acidic than H, because loss of H, forms a resonance-stabilized conjugate base. H, is less acidic than H, because loss of H, forms a resonance-stabilized conjugate base. H, is more acidic than H, because loss of H, forms a resonance-stabilized conjugate base. a. H, is less acidic than H, because loss of H, forms a resonance-sta bilized conjugate base.arrow_forwardHow do you draw this structure's conjugate base?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

