
Concept explainers
Interpretation: Any one structure from top row is enough to describe the molecule in top row but all structure is needed from bottom row to describe the molecule in bottom row should be explained.
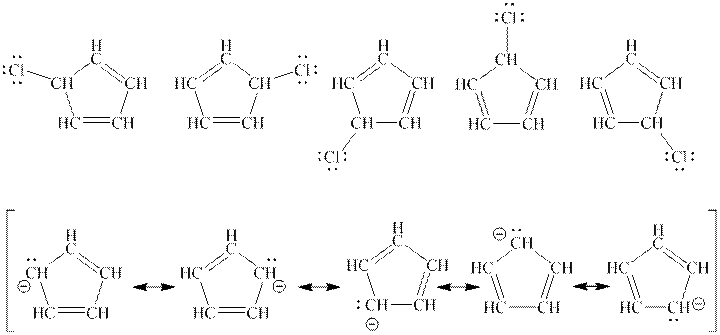
Concept introduction: When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Which of the following choices depicts the correct resonance arrows to show the conversion between the Q resonance form to the R resonance form?arrow_forwardWhat are four resonance structures of this molecule and which ones are major and minorarrow_forwardWhat are fuctional groups, are they only attached to carbon molecules or molecules that don't contain carbon?arrow_forward
- The structure at the right is a skeleton of an anion having the overall formula C6H,NO¯. The hydrogen atoms are not shown. (a) Draw a complete Lewis structure in which the -1 formal charge is on N. Include all H atoms and C. valence electrons. (b) Do the same for a Lewis structure with the -1 formal charge on O. (c) Do the same for a Lewis structure with the -1 formal charge on the C atom that is bonded to three other C atoms.arrow_forwardA resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons.arrow_forwardWhich of the following species is a valid resonance structure of A? Usecurved arrows to show how A is converted to any valid resonancestructure. When a compound is not a valid resonance structure of A,explain why not.arrow_forward
- A resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part3: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all nonzero formal charges and all nonbonding electrons.arrow_forwardIn the following Lewis structure of [SiF6]², every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). :F: :F : :ד: Si 1 :F: 0 0 0 2+ :H: Ë: 0 2-arrow_forwardDraw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons.arrow_forward
- Doxorubicin, shown here, is an important chemotherapy drug used to treat avariety of cancers, including bladder cancer, breast cancer, and certain forms of leukemia. Doxorubicin works by binding to DNA in such a way that a portion of it penetrates the DNA double helix— a process called intercalation. During transcription— the process that forms RNA— portions of the DNA strands are temporarily separated for the base sequence to be read and then are reconnected. With bound doxorubicin, however, the double helix does not reform properly after the strands are separated, which disrupts replication— the process that forms an identical copy of DNA. Which portion of doxorubicin do you think intercalates into the DNA double helix, and why do you think it has little difficulty doing so?arrow_forwardDraw Lewis structures for each of the following ions. One atom in each ion has a formal charge that is not zero.Determine which atom it is, and what the formal charge is. (a) the C2H5 anion; (b) the CH3O cation; (c) the CH6N cation;(d) the CH5O cation; (e) the C3H3 anion (all three H atoms are on the same carbon)arrow_forwardDraw a Lewis structure of your choice that has at least three equally reasonable resonance structures. You must show your work as to how the structure was drawn including showing valence electrons, any structures you draw that don’t work and require you to show a multiple bond, and formal charges on each atom that has a formal charge. For example, Nitrate ion has three structures (but you can’t draw this one). All three structures have formal charges and no one structure is preferred based on formal charge. Carbon dioxide would not be a valid choice. While it does have three structures, the structure that has two double bonds is the preferred structure while the structures with triple bonds are not equivalent to the double bonded version as they have multiple formal charges present.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

