
Concept explainers
Interpretation: The Lewis structure of below
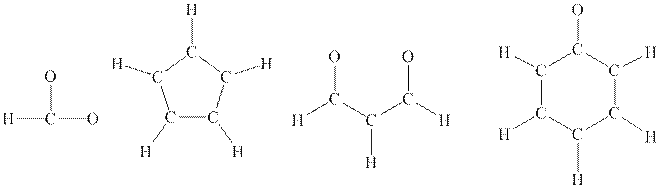
Concept introduction: Lewis structure is representation of molecule in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Provide all reasonable resonance structures for each molecular structure below. Use curved arrows to show how electrons are moved and dont forget lone pairs. E 1. CH3 CNO E2. CHỌN -1 NCO E3. CHOCN E +1 4. O C S E5. N = N = O E1. CH CNO E2. CH3NCO E3. CH₂OCN E4. O-C-S E5. N-1=N+1=0 E6. CH H₂Oarrow_forwardcan someone explain how Molecule B has a tertiary carbon ? Im having troubl with secondary and tertiary i tried to calculate the formal charges thinking that may have something to do with it but I ended up getting the wrong formal charge for molecule B, so now I'm confused.arrow_forwardDraw all reasonable resonance structures for each species. a. O3 c. Ng e. g. :O: :O: b. NO, (a central N atom) d. CH3-C-CH-C-CH, 1. CH2=CH-CH-CH=CH2arrow_forward
- In the following Lewis structure of [(CH3)2OH]*, every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). H. H-C Н-С-О-С-Н C-H H HH 2- II 2-arrow_forward3. Which of the following molecules are conjugated? For those which are conjugated, write a resonance structure.arrow_forward2. Draw the two resonance structures of benzene (nose 1q T)arrow_forward
- Four major contributing resonance structures are possible for the given cation, which is the intermediate o complex of an electrophilic aromatic substitution involving phenol and bromine. Two structures are given but are incomplete. Complete the given structures by adding nonbonding electrons and formal charges. Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Complete structure A. Complete structure B. : 0 H :0- Br : Brarrow_forwardI am unsure of how to draw all the available resonance structures for these structures. Could anyone help please? Thank you.arrow_forward15. Draw all the important resonance structures for the following ion, and show all the lone pair of electrons and formal charges. Show the electron flow by using arOws. NH NH 16. Draw skeletal structures of compounds accordinghto he rules provided and write their common names 100 16b. drawskeletal 16c. draw skeletal 16d drawskeletal arawskeletal an ether that contains abenzer ing (No limit on the total rumber of carbons) a secondary alkyl chloride with 4 carbons total a primary alcohol with 4 carbons total a tertiary amine with 6 carbons total common name common name common name common namearrow_forward
- Resonance, hybridization, Lewis structures: Draw the most important resonance contributor of the butene-1 ion Draw the resonance form of the following structure. Interactive 3D display mode H₂C Draw the structure on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. NN [1] A CH₂ H: 2D EXP. CONT L Marvin JS by ChemAxon H C N O S CI Br - P LL Farrow_forwardDraw the best Lewis structure for the linear C ion, including all lone pair electrons and non-zero formal charges.arrow_forwardDraw two additional resonance structures of the following. Remember to include all formal charges needed. Clearly show the arrows to go from one structure to the next.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


