
Concept explainers
(a)
Interpretation: The missing resonance structures for anion shown below should be determined.
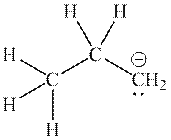
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(b)
Interpretation: The missing resonance structures for anion shown below should be determined.
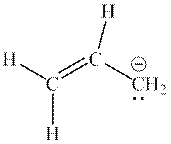
Concept introduction: When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(c)
Interpretation: The missing resonance structures for anion shown below should be determined.
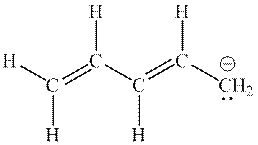
Concept introduction: When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Which of the following shows correct arrow placement that represents significant resonance for the given molecule? None of the choices given represent significant resonance. Save for Laterarrow_forwardHow many total resonance structures can be drawn for the following anion (include those without separation of charge)?arrow_forwarddraw all the significant resonance structures of this moleculearrow_forward
- Draw a reasonable resonance structure for the following species. Draw out all bonds to the oxygen atom in your answer.arrow_forwardDraw all reasonable resonance structures for the following compounds. Be sure to show the proper arrows to indicate electron movementarrow_forwardDraw all possible resonance forms for anisole using appropriate arrow notation. Which resonance structure is most stable? Which is least stable? Draw the resonance hybrid for anisole, indicating all partial charges.arrow_forward
- For each of the following molecules, draw all the valid resonance formsarrow_forwardFor each of the following molecules, complete the following. (Please Explain) Draw the bond dipole for each polar bond. Indicate whether there is a net dipole or not using the blanks provided. You don’t need to provide the direction of the net dipole, only whether or not one exists.arrow_forwardWhich of the highlighted chemical bonds in the molecules below is longest? Shortest? In between? Which highlighted bond requires the highest energy to break? Lowest? In between? Answer these questions by completing the second and third columns in the table.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


