
Concept explainers
(a)
Interpretation:The resonance structure that results from curved arrows mentioned below should be determined.
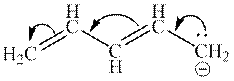
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(b)
Interpretation:Whether the use of arrow type 3 in resonance structure of anion skips the important resonance structure or not should be determined.
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(c)
Interpretation: The curved arrowsin the resonance structure mentioned below should be added.
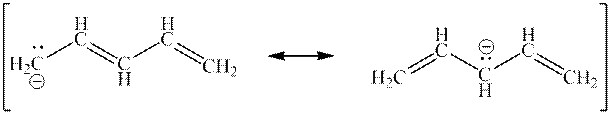
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- For each proposed set of resonance structures: a. (E) Add curved arrows (starting from left) to show how each successive r.s. was generated. b. Cross out any resonance structures that are NOT important, and explain your reasoning.arrow_forwarda model of each molecule shown above: Is the molecule in the left box the same moleculeas the molecule in the right box? Use your models to answer the question, and recall that...arrow_forwardSee attached image. For this molecule draw the 2 best resonance structures in the boxes. Circle the best.arrow_forward
- Three major contributing resonance structures are possible for the anion NO,. One resonance structure is shown, but is Add missing charges and non-bonding electrons. incomplete. Complete the given structure by adding non-bonding electrons and formal charges. Draw the remaining structures, including non-bonding electrons and Select Draw Rings More Erase N formal charges. Do not draw curved arrows. || N. Draw major resonance structure 2. Be sure to add Draw major resonance structure 3. Be sure to add charges and non-bonding electrons where appropriate. charges and non-bonding electrons where appropriate. Select Draw Rings More Erase Select Draw Rings More Erase N Narrow_forwardx-xo B Draw molecule A. On that drawing include the lone pairs and the curved arrows that would produce resonance structure B.arrow_forward1. Draw the best possible resonance contributor. Draw curved arrows to show electron movement.arrow_forward
- Is this a valid resonance structure? why or why not. (the bond is moved to the left) forgot to include arrowthank you for your time.arrow_forwardTrue or False? Circle your answer.a. A resonance hybrid is a structure with equal contribution from each possible resonance structure.True Falseb. Localized electrons do not participate in resonance.True Falsearrow_forwardI am having trouble on part c. It wants me to draw the resonance structure, but in mastering chem there’s no arrows available for some reason . Is there a way to draw it without the arrows and still be resonancearrow_forward
- True or False? Circle your answer. a. The sum of all formal charges on a molecule is equal to the charge on the molecule or ion. True or False b. If the total charge on a molecule is zero, then each atom in the molecule has a formal charge of zero. True or Falsearrow_forwardBecause this is a charged species, let's focus on resonance patterns that can delocalize the charge. First, add curved arrow(s) to show the resonance using the following pattern: a lone pair next to a pi bond. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the+ and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. NH Edit Drawing *NHarrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. The target molecule. H H Note for advanced students: what we mean by "cutting" the bond here is breaking the bond and attaching H atoms to each dangling end, like this: ++*++ H C-H H-ő-H H X -Ö-H Sarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
