
Concept explainers
Interpretation: The reason that explains zero possibility for resonance structure of following anion should be determined.
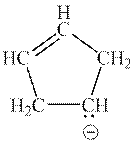
Concept introduction: Lewis structure is a representation of molecules in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of a single-molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for the resonance structure of anions in the movement of negative charge.
2. Use only arrow type 3 to move a positive charge for the resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be the same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Some of the figures below represent invalid sets of resonance structures for this molecule.arrow_forward1. Molecule: CF4 indicate the number of available electrons that electrons ae = are in the molecule. in the space to the right connect all of the atoms to the central atom and then make each atom follow the octet rule Trial Structure: (duet rule for hydrogen). How many electrons are necessary in the trial ne = structure? Circle the correct ne = ae ne ae relationship between ne and ae. Draw the corrected Lewis Structure to the right. Add Later: e- geometry: molecular geOm Hybridization:arrow_forwardDraw the Lewis structures for the following four molecules, being sure to show all steps following the methods covered in class. Structures without work shown will be marked incorrect. Also, one of these molecules has resonance structures – for this compound, make sure to include all resonance structures, indicate formal charges for each atom. SO2 OF2 IF3 NH4+ Consider a molecule where the central atom has one lone pair of electrons and is double-bonded to two other atoms (of a different element). Draw a general diagram of the molecule. Is this molecule likely a polar or nonpolar molecule? Briefly explain your reasoning, using words and the diagram.arrow_forward
- Some of the figures represent invalid sets of resonance structures for this molecule. Select all the VALID sets of resonance structures.arrow_forwardWhat is the name of this type of molecular structure?? I need help answering this question because I don’t know what format it’s calledarrow_forwardWhat is the formal charge on chlorine in the best Lewis structure for chloric acid? type your answer...arrow_forward
- Determine if each of the following is a valid Lewis structure based on the information given at the beginning of the introduction. If it is valid, simply write valid under the structure. If it is not valid, explain why.arrow_forwardHow do you know when to draw a solid wedge vs a dashed wedge when drawing 3D bond-line structures? I know that solid-wedge means the atom is pointing towards you and dashed wedge means it's in the back, but how do you know which atoms are in the front as opposed to the back? How can you tell what the configuration will look like in space just by looking at the lewis structure or name?arrow_forwardThe same student concluded by saying “Bond type can be calculated by subtracting the electronegativities of the elements involved in a chemical bond. Electronegativity differences below 1.7 are covalent bonds, and electronegativity differences 0.4 and below are non-polar covalent bonds and between 0.4 to 1.6 are considered by most to be polar covalent bonds.” Do you agree with this student? Justify your response.arrow_forward
- Explaine why, in Part I, the formal charge is equal to the number of valence electrons. Match the words in the left column to the appropriate blanks in the sentences on the right. minus The formal charge on each atom is the number of valence electrons the number of plus lone pair electrons and the number of bonding electrons. The formal charge is equal to the number of valence electrons because there are bonds. one-half two times no multiplearrow_forwardClear my choice With regard to resonance structures that are drawn for a real species, which of the following statements is NOT TRUE? Select one: A. Resonance structures all have electron localized structures, while the real species that is described by them has delocalized electrons. OB. Resonance structures are real molecules that co-exist in equilibrium with each other. O C. Resonance structures differ in the location of their electrons, not the positions of their atoms. O D. Resonance structures are only drawings; each structure alone does not represent an existing molecule. Clear my choice What type of hybrid orbitals is used by the nitrogen atom in the following molecule? HO-N=0: hparrow_forwardIV. Write the resonance structure that would result from moving the electrons in the way indicated by the curved arrows. Submit your answer as assignment. H2Narrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
