
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 24CTQ
Interpretation Introduction
Interpretation: Weaker and stronger base on the basis of
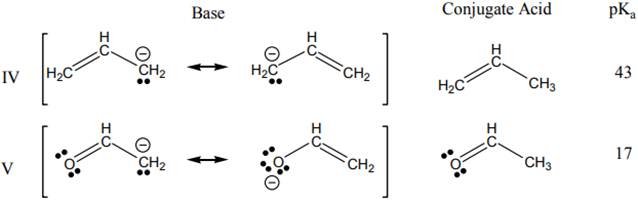
Concept introduction: The dissociation constant of acid represents strength of acid in solution. It is denoted by
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a. Which of the acids in the table above has the strongest conjugate base?
b. What is the structure of the strongest acid in the table above?
Consider cyclohexane and benzene (draw the structures below). Draw the conjugate base of each molecule and then explain why cyclohexane is the weaker acid.
Which is a stronger base, S2 - or Se2 -? Explain.
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 5 - Which elements on the periodic table (other than...Ch. 5 - You will not find “hydroxide” in the stockroom,...Ch. 5 - Prob. 3CTQCh. 5 - Prob. 4CTQCh. 5 - Prob. 5CTQCh. 5 - Prob. 6CTQCh. 5 - On which do you expect to have a more intense and...Ch. 5 - Prob. 8CTQCh. 5 - Prob. 9CTQCh. 5 - Prob. 10CTQ
Ch. 5 - Prob. 11CTQCh. 5 - Prob. 12CTQCh. 5 - Prob. 13CTQCh. 5 - Prob. 14CTQCh. 5 - Prob. 15CTQCh. 5 - Prob. 16CTQCh. 5 - For each proposed set of resonance structures: a....Ch. 5 - Consider the polarization of the C=O bond in the...Ch. 5 - The C=O double bond is called a “carbonyl bond.”...Ch. 5 - Prob. 20CTQCh. 5 - Prob. 21CTQCh. 5 - Prob. 22CTQCh. 5 - Prob. 23CTQCh. 5 - Prob. 24CTQCh. 5 - Prob. 25CTQCh. 5 - Prob. 26CTQCh. 5 - Prob. 27CTQCh. 5 - Prob. 28CTQCh. 5 - Prob. 29CTQCh. 5 - Prob. 30CTQCh. 5 - Prob. 31CTQCh. 5 - Confirm that there is no legitimate Lewis...Ch. 5 - Draw all resonance structures of the molecule...Ch. 5 - Prob. 34CTQCh. 5 - Prob. 35CTQCh. 5 - Prob. 36CTQCh. 5 - Occasionally, we will see an ionic compound that...Ch. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Is it possible to draw a resonance structure of...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - Phenol (shown below) has a pKa10 . a. Based on pKa...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Prob. 12ECh. 5 - Complete each Lewis structure, draw all important...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Construct an explanation for why sulfuric acid is...Ch. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - Prob. 18E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forwardFor each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forwardThe following are equivalent ways of asking about the acidity of an H atom: • What is the most acidic H on the molecule? • Which H is associated with the published pKa value? • Which H on the molecule is easiest to remove? • Which H on the molecule takes the least energy to remove? • Which bond to an H is most polarized? • For which H atom is removal least uphill in energy? • Which bond to an H atom, when broken, results in the lowest PE conjugate base? We will often find the last of these questions is easiest to answer. To do this, find all the different Hatoms on the molecule, and draw all possible conjugate bases.Only the lowest-energy one is the “real” conjugate base. Identify this structure, and you have found the most acidic H. Use this strategy to find the most acidic H on each of the following molecules. Note: Each structure hasat least three different kinds of H’s, so draw at least three unique conjugate bases for each.arrow_forward
- For the previous four questions, label each molecule that appears in the question or your answer asstrong acid, strong base, weak acid, or weak base.arrow_forwardFor each equation, label the Lewis acid and the Lewis base. In addition, show all unshared pairs of electrons on the reacting atoms and use curved arrows to show the flow of electrons in each reaction. (a) F + BF3 BF4arrow_forwardAnswer true or false to the following statements about the mechanism of acid-base reactions. (a) The acid and base must encounter each other by a collision in order for the proton to transfer. (b) All collisions between acids and bases result in proton transfer. (c) During an acid-base reaction the lone pair on the base fills the A-H antibonding sigma orbital.arrow_forward
- Several acids and their respective equilibrium constants are: Which is the strongest acid? Which is the weakest acid? Which acid has the weakest conjugate base? Which acid has the strongest conjugate base?arrow_forwardRank the following conjugate bases in order of decreasing basicity, putting the most basic first. H2C=CH HC=C CH3 II Multiple Choice || > |> |II |> || > III III > || > | III > | > ||arrow_forwardRank the bases in the table below from strongest (1) to weakest (4).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning