
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
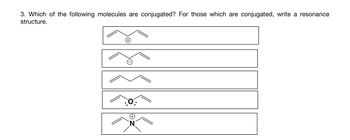
Transcribed Image Text:3. Which of the following molecules are conjugated? For those which are conjugated, write a resonance
structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Oo.37. Subject :- Chemistryarrow_forwardOne, or both, of the following structural formulas may be incorrect (i. e., they do not represent a real compound) because they have atoms with an incorrect number of bonds. Which of the labeled atoms have the incorrect number of bonds? Specify the incorrect atoms by letter in alphabetical order without spaces or commas., i.e. bd. If there are no incorrect atoms, write none. b c H-N=C-0=c-H a a) H H H od b) C-H Harrow_forwardNitromethane is an organic compound with the molecular Draw a complete Lewis structure for the conjugate acid of nitromethane that shows all bonds, unshared electron pairs, and minimized formal charges, where appropriate. formula CH, NO,. Draw a complete Lewis structure that shows all bonds, unshared electron pairs, and minimized formal charges, where appropriate. H H 1. H : 0 :arrow_forward
- Part 2 Provide the structure for Compound X in the reaction scheme below. Include all lone pairs and formal charges. X÷F•Y÷F Compound X + D + ос LDA -78 °C 2D H 1.-78 °C 2. NH4Cl Compound Z See Hint H C N O S F Parrow_forwardouro- 6)- Draw the structures for the least and most polar molecules (i.e.,isomers) of Bromo-chloro-fl iodoethene (C2BrCIFI). Briefly explain your choice. Structure of least polar: Structure of most polar:arrow_forwardPlease see attachedarrow_forward
- Draw the skeletal (line-bond) structure of [CH₃CH₂CHC(O)CH₂CH₂CH₃]⁻. Include all lone pairs and charges as appropriate. What do the brackets and the - exponent mean for this formula?arrow_forward9. Draw all resonance structures of the following compounds. Show electron-pushing arrows.arrow_forwardProvide the following 2 resonance structures and indicate whether or not they're major or minorarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY