
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.33P
Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction.
(a) 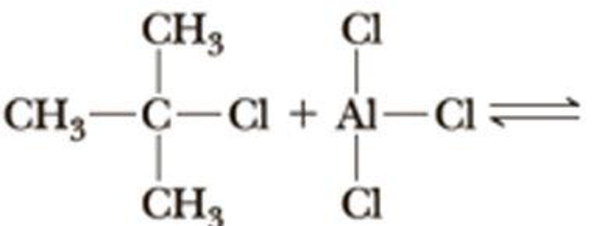
(b) 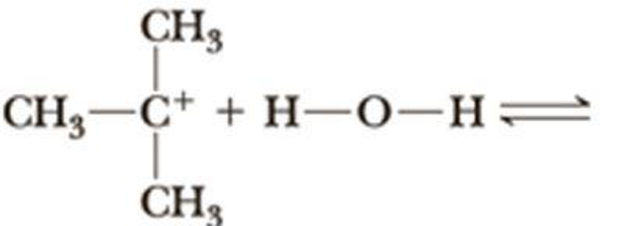
(c) 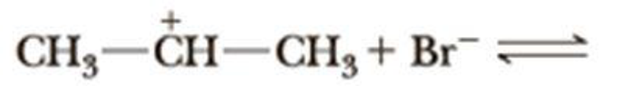
(d) 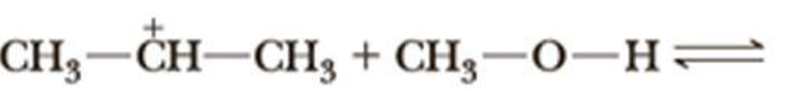
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Consider the following reaction: (a) What is the acid on the left side of the equation? (b) What is the base on the left side of the equation? (c) What is the conjugate base of the acid on the left? (d) What is the conjugate acid of the base on the left? (e) What is the acid on the right side of the equation? (f) What is the base on the right side of the equation? (g) What is the conjugate base of the acid on the right? (h) What is the conjugate acid of the base on the right?
Explain using examples how electronic factors (resonance and inductive effects) can be
used to determine acidity and basicity of organic compounds.
Use electronic and stability factors to account for the acidity of each highlighted hydrogen in
the molecule below. Identify the most acidic proton.
(a)
HO.
(b)
HO.
(c)
H.
complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction
Chapter 4 Solutions
Organic Chemistry
Ch. 4.2 - For each conjugate acid-base pair, identify the...Ch. 4.2 - Write these reactions as proton-transfer...Ch. 4.2 - Following is a structural formula for guanidine,...Ch. 4.2 - Write an equation to show the proton transfer...Ch. 4.3 - For each value of Ka, calculate the corresponding...Ch. 4.4 - Predict the position of equilibrium and calculate...Ch. 4.5 - Calculate Keq for a reaction with G0 = 17.1 kJ/mol...Ch. 4.6 - Acid-Base Equilibria Many factors contribute to...Ch. 4.6 - What is the relative trend in acidity and pKa of...Ch. 4.7 - Write an equation for the reaction between each...
Ch. 4 - For each conjugate acid-base pair, identify the...Ch. 4 - Complete a net ionic equation for each...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - Prob. 4.12PCh. 4 - In acetic acid, CH3COOH, the OH hydrogen is more...Ch. 4 - Which has the larger numerical value? (a) The pKa...Ch. 4 - In each pair, select the stronger acid. (a)...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - If the G for a reaction is 4.5 kcal/mol at 298 K,...Ch. 4 - Calculate the Keq for the following reactions from...Ch. 4 - Prob. 4.20PCh. 4 - Answer true or false to the following statements...Ch. 4 - In each of the following three reaction coordinate...Ch. 4 - The acid-base chemistry reaction of barium...Ch. 4 - Unless under pressure, carbonic acid (H2CO3) in...Ch. 4 - Prob. 4.25PCh. 4 - Acetic acid, CH3COOH, is a weak organic acid, pKa...Ch. 4 - Benzoic acid, C6H5COOH (pKa 4.19), is only...Ch. 4 - Prob. 4.28PCh. 4 - One way to determine the predominant species at...Ch. 4 - Will acetylene react with sodium hydride according...Ch. 4 - Prob. 4.31PCh. 4 - For each equation, label the Lewis acid and the...Ch. 4 - Complete the equation for the reaction between...Ch. 4 - Each of these reactions can be written as a Lewis...Ch. 4 - The sec-butyl cation can react as both a...Ch. 4 - Prob. 4.36APCh. 4 - Prob. 4.37APCh. 4 - Prob. 4.38APCh. 4 - Explain why the hydronium ion, H3O+, is the...Ch. 4 - What is the strongest base that can exist in...Ch. 4 - Prob. 4.42APCh. 4 - Prob. 4.43APCh. 4 - Methyl isocyanate, CH3N=C=O, is used in the...Ch. 4 - Offer an explanation for the following...Ch. 4 - Prob. 4.46APCh. 4 - Alcohols (Chapter 10) are weak organic acids, pKa...Ch. 4 - As we shall see in Chapter 19, hydrogens on a...Ch. 4 - 2,4-Pentanedione is a considerably stronger acid...Ch. 4 - Write an equation for the acid-base reaction...Ch. 4 - Prob. 4.51APCh. 4 - Prob. 4.52APCh. 4 - Prob. 4.53APCh. 4 - Following is a structural formula for imidazole, a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the position of equilibrium for this acid-base reaction.arrow_forwardAnswer true or false to the following statements about the mechanism of acid-base reactions. (a) The acid and base must encounter each other by a collision in order for the proton to transfer. (b) All collisions between acids and bases result in proton transfer. (c) During an acid-base reaction the lone pair on the base fills the A-H antibonding sigma orbital.arrow_forwardRank the Acidity of the following sets of compounds. Give an explanation for the answers.arrow_forward
- complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reactionarrow_forwardIf 0.100 mol of phenol, C6H5OH, were dissolved in pure water to make 1.000 L of total solution, what would the concentration of C6H5O- be at equilibrium? What would the acid’s percent dissociation be?arrow_forward2.) Draw the products of the following reactions. Provide an arrow-pushing mechanism for each reaction. Label the acid, base, conjugate base, and conjugate acid. Finally, indicate the direction of equilibrium. 요요 + NaHarrow_forward
- For each conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate acid or base. In addition, draw Lewis structures for each species, showing all valence electrons and any formal charge. (a) CH3CH3 CH3CH2-arrow_forwardConsider cyclohexane and benzene (draw the structures below). Draw the conjugate base of each molecule and then explain why cyclohexane is the weaker acid.arrow_forward4. The pk, of vanillin is about 9, which is much more acidic than a normal alcohol. Draw a reaction showing the deprotonation of vanillin with NaOH, and then draw six resonance structures of the conjugate base. Draw the hybrid structure and clearly indicate how the negative charge is distributed in the compound.arrow_forward
- Which species is the stronger Lewis Acid? Why? (Use resonance structures to explain your answer.) BH3 or trimethoxyboratearrow_forwardAs we will see in later chapters, many steps in key reaction sequences involve acid–base reactions. (a) Draw curved arrows to illustrate the flow of electrons in steps [1]–[3]. (b) Identify the base and its conjugate acid in step [1]. (c) Identify the acid and its conjugate base in step [3].arrow_forwardcomplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY