
Interpretation: To propose a scheme for the production of Ethylene glycol.
Concept Introduction: Ethylene glycol is a
Answer to Problem 70A

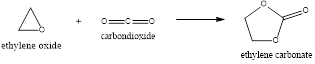
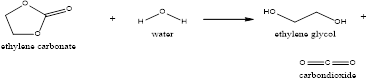
Explanation of Solution
In the first step, the ethylene is made to react with the oxygen resulting in the formation of ethylene oxide. This reaction is an exothermic reaction that takes place in the gaseous phase with the help of some catalysts like silver that is supported on alumina.

The ethylene oxide produced is made to react with the carbon dioxide resulting in the formation of ethylene carbonate and this ethylene carbonate produced is hydrolyzed to form the mono-ethylene glycol and also carbon dioxide.
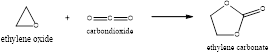
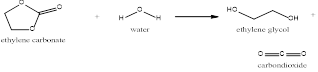
In the above process, ethylene glycol which is also called Mono-ethylene glycol is produced from ethylene oxide. This process is the conversion of ethylene oxide to ethylene glycol in the presence of catalysts.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





