
a)
Interpretation: To write the expected product that is obtained by the given reactions.
Concept Introduction: Oxidation reactions involve the transfer of elements between two elements. The oxidation number of an atom or an ion gets changed during the oxidation reaction. Oxidation is also referred to as the reaction of gaining oxygen.
a)
Answer to Problem 43A
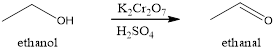
Explanation of Solution
In the given reaction the primary alcohols are converted to
b)
Interpretation: To write the expected product that is obtained by the given reactions.
Concept Introduction: Oxidation reactions involve the transfer of elements between two elements. The oxidation number of an atom or an ion gets changed during the oxidation reaction. Oxidation is also referred to as the reaction of gaining oxygen.
b)
Answer to Problem 43A
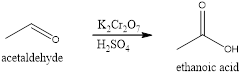
Explanation of Solution
In the given reactions, aldehydes are converted to
c)
Interpretation: To write the expected product that is obtained by the given reactions.
Concept Introduction: Oxidation reactions involve the transfer of elements between two elements. The oxidation number of an atom or an ion gets changed during the oxidation reaction. Oxidation is also referred to as the reaction of gaining oxygen.
c)
Answer to Problem 43A
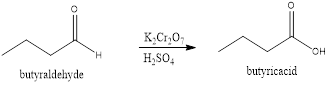
Explanation of Solution
In the given reaction, the aldehydes are converted to carboxylic acids. The conversion of these aldehydes will take place in the presence of
d)
Interpretation: To write the expected product that is obtained by the given reactions.
Concept Introduction: Oxidation reactions involve the transfer of elements between two elements. The oxidation number of an atom or an ion gets changed during the oxidation reaction. Oxidation is also referred to as the reaction of gaining oxygen.
d)
Answer to Problem 43A
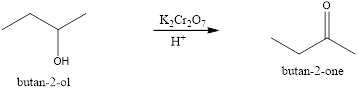
Explanation of Solution
In the given reaction, the alcohol is given as the secondary alcohol. The oxidation of the secondary alcohol in the presence of
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





