
a.
Interpretation: To write the names and structures of the products that are obtained by the reaction of ethene with the following reagents.
Concept Introduction: Addition reactions are those in which two or more reactants combine to form a product. Addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water,
a.
Answer to Problem 38A
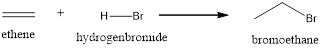
Explanation of Solution
The above reaction is an electrophilic addition reaction which is useful for the preparation of
b.
Interpretation: To write the names and structures of the products that are obtained by the reaction of ethene with the following reagents.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
b.
Answer to Problem 38A
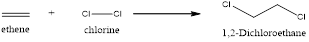
Explanation of Solution
In this reaction, the pi of the alkene and the sigma bond of the alkene will break resulting in the formation of a new bond. This reaction will follow Markonikov’s rule. In the first step, the double bond of the alkene breaks and results in the formation of a carbocation. In the second step, the bond between the halogen breaks and gets attached to the carbocation. Now the halogen will get attached to the carbocation resulting in the formation of 1,2-dichloroethane.
c.
Interpretation: To write the names and structures of the products that are obtained by the reaction of ethene with the following reagents.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
c.
Answer to Problem 38A

Explanation of Solution
The hydration reaction of an alkene is an example of an electrophilic addition reaction. The double bond in the alkene which is ethene will act as a nucleophile and the acidic proton will act as an electrophile. The first step will result in the formation of a carbocation. The second step involves the addition of a nucleophile to the carbocation resulting in the formation of alcohol.
d.
Interpretation: To write the names and structures of the products that are obtained by the reaction of ethene with the following reagents.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
d.
Answer to Problem 38A

Explanation of Solution
This reaction takes place in the presence of a catalyst. The pi-bond of the alkene will act as a source of electrons for electrophiles. The bond between the hydrogen breaks in the presence of the catalyst resulting in the formation of the metal-hydrogen bond. The surface of the metal absorbs the alkene and helps in adding the other hydrogen resulting in the formation of a saturated compound.
e.
Interpretation: To write the names and structures of the products that are obtained by the reaction of ethene with the following reagents.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
e.
Answer to Problem 38A
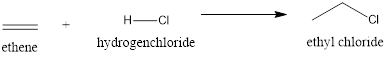
Explanation of Solution
The above reaction is an electrophilic addition reaction which is useful for the preparation of alkyl halides. In this, the first step involves the formation of the carbocation that will rearrange to a stable form. In the second step, the chlorine will attack the carbocation resulting in the formation of ethyl chloride in this case.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





