
Interpretation: To predict the product of acid-catalyzed reaction hydrolysis of an ester.
Concept Introduction: Catalysis is added to increase the rate of a
Answer to Problem 1STP
Alcohols
Explanation of Solution
Acid-catalyzed hydrolysis of ester will take place through a unimolecular nucleophilic substitution reaction. The acid which acts as a catalyst in this reaction protonates the carbonyl carbon of the ester making it liable for the nucleophilic attack.
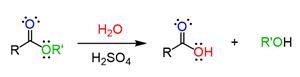
In the above reaction, the ester will undergo protonation and makes the carbonyl carbon more electrophilic making it liable for the attack of the nucleophile. The lone pair of electrons that are present on the oxygen atom of the water molecule will attack the electrophilic center and then result in the formation of an intermediate. This intermediate will undergo further reaction to produce
This acid-catalyzed reaction is reversible. The catalyst used here is used to increase the rate of the chemical reaction and the lone pairs present in the oxygen of ester will also participate in the reaction. The product is formed by protonation and nucleophilic attack.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





