
a)
Interpretation: To identify the
Concept Introduction: The compounds given are represented by the ball and stick model. In this model, the atoms are represented with balls, and the bonds are represented by sticks. Each atom present in the molecule will be represented with a different color in order to differentiate them.
a)
Answer to Problem 53A
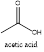
The functional group present is a
Explanation of Solution
In the given ball and stick model, the carbons are represented with black color, the hydrogens are represented with white and the oxygen is represented with red color. This clearly shows that the functional group present is a carboxylic acid.
b)
Interpretation: To identify the functional group and the names of the compounds given.
Concept Introduction: The compounds given are represented by the ball and stick model. In this model, the atoms are represented with balls, and the bonds are represented by sticks. Each atom present in the molecule will be represented with a different color in order to differentiate them.
b)
Answer to Problem 53A
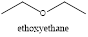
The functional group present is ether and the name of the compound is ethoxyethane.
Explanation of Solution
In the given ball and stick model, the carbons are represented with black color, the hydrogens are represented with white and the oxygen is represented with red color. This clearly shows that the functional group present is ether.
c)
Interpretation: To identify the functional group and the names of the compounds given.
Concept Introduction: The compounds given are represented by the ball and stick model. In this model, the atoms are represented with balls, and the bonds are represented by sticks. Each atom present in the molecule will be represented with a different color in order to differentiate them.
c)
Answer to Problem 53A

The functional group present is a
Explanation of Solution
In the given ball and stick model, the carbons are represented with black color, the hydrogens are represented with white and the oxygen is represented with red color. This clearly shows that the functional group present is a ketone and the compound is a carbonyl compound.
d)
Interpretation: To identify the functional group and the names of the compounds given.
Concept Introduction: The compounds given are represented by the ball and stick model. In this model, the atoms are represented with balls, and the bonds are represented by sticks. Each atom present in the molecule will be represented with a different color in order to differentiate them.
d)
Answer to Problem 53A

The functional group present is alcohol and the name of the compound is ethanol.
Explanation of Solution
In the given ball and stick model, the carbons are represented with black color, the hydrogens are represented with white and the oxygen is represented with red color. This clearly shows that the functional group present is alcohol.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





