
a.
Interpretation: To write the IUPAC names of the ethers that are given.
Concept Introduction: The nomenclature of ethers follows some rules to name the compound. The alkyl groups and the aryl group that is attached on either side of the oxygen are named alphabetically by adding ether at the end. The substituent group with a greater number of carbons is considered as parent hydrocarbon and the other substituent group attached to the same oxygen is named using the ‘oxy’.
a.
Answer to Problem 40A
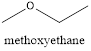
Explanation of Solution
In the above example, the shorter chain is containing only one carbon and the longest chain contain two carbons. Since the shorter chain ends with oxy followed by the name of the longest carbon chain which is the parent hydrocarbon chain the name is given as methoxy ethane.
b.
Interpretation: To write the IUPAC names of the ethers that are given.
Concept Introduction: The nomenclature of ethers follows some rules to name the compound. The alkyl groups and the aryl group that is attached on either side of the oxygen are named alphabetically by adding ether at the end. The substituent group with a greater number of carbons is considered as parent hydrocarbon and the other substituent group attached to the same oxygen is named using the ‘oxy’.
b.
Answer to Problem 40A
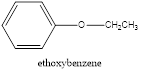
Explanation of Solution
In the above example, the shorter chain is containing only two carbon and the longest chain contain six carbons. Since the shorter chain ends with oxy followed by the name of the longest carbon chain which is the parent hydrocarbon chain the name is given as ethoxy benzene.
c.
Interpretation: To write the IUPAC names of the ethers that are given.
Concept Introduction: The nomenclature of ethers follows some rules to name the compound. The alkyl groups and the aryl group that is attached on either side of the oxygen are named alphabetically by adding ether at the end. The substituent group with a greater number of carbons is considered as parent hydrocarbon and the other substituent group attached to the same oxygen is named using the ‘oxy’.
c.
Answer to Problem 40A

Explanation of Solution
In the above example, the number of carbons is the same on both sides of the oxygen. Any side can be considered as a shorter chain and the other side as a longer chain. There is a presence of a vinyl group in the given structure therefore, the name is given as vinyl oxy ethene.
d.
Interpretation: To write the IUPAC names of the ethers that are given.
Concept Introduction: The nomenclature of ethers follows some rules to name the compound. The alkyl groups and the aryl group that is attached on either side of the oxygen are named alphabetically by adding ether at the end. The substituent group with a greater number of carbons is considered as parent hydrocarbon and the other substituent group attached to the same oxygen is named using the ‘oxy’.
d.
Answer to Problem 40A
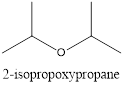
Explanation of Solution
In the above example, there is an isopropyl group on both sides of the oxygen. If one side is considered a short chain then the other side will have three carbons in the longer chain. Therefore, the name is 2-isopropoxypropane.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





