
a.
Interpretation: To give the IUPAC names of the given alcohols.
Concept Introduction: The
a.
Answer to Problem 37A
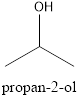
Explanation of Solution
In the given compound, the longest chain contains three carbons and the hydroxyl group is located on the second carbon. Therefore, it is named Propan-2-ol.
b.
Interpretation: To give the IUPAC names of the given alcohols.
Concept Introduction: The IUPAC nomenclature of alcohols will begin with the selection of the longest carbon chain with an attached hydroxyl group. Number the longest carbon chain in such a way that the hydroxyl group will get the least number. Name the substituent first and then add the longest carbon chain along with the suffix “ol”.
b.
Answer to Problem 37A
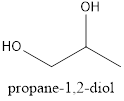
Explanation of Solution
In the given compound, the longest chain contains three carbons and the hydroxyl group is located on the first and the second carbon. Therefore, it is named Propan-1,2-
c.
Interpretation: To give the IUPAC names of the given alcohols.
Concept Introduction: The IUPAC nomenclature of alcohols will begin with the selection of the longest carbon chain with an attached hydroxyl group. Number the longest carbon chain in such a way that the hydroxyl group will get the least number. Name the substituent first and then add the longest carbon chain along with the suffix “ol”.
c.
Answer to Problem 37A
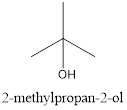
Explanation of Solution
In the given compound, the longest chain contains three carbons and the hydroxyl group is located on the second carbon. Along with the hydroxyl group, there is a methyl group present at the second carbon. Therefore, it is named 2-methylpropan-2-ol.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





