
Interpretation: To write the structures of the alcohols that are oxidized to give each carbonyl compounds that are mentioned.
Concept Introduction: Alcohols are converted to carbonyl compounds by a process called oxidation. This oxidation takes place in the presence of oxidizing agents and some catalysts. Oxidation is generally the loss of hydrogen. Therefore, alcohols lose hydrogen to form carbonyl compounds.
Answer to Problem 54A
Methanol will produce Formaldehyde on oxidation. The structure is
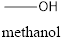
Isopropyl alcohol will produce acetone on oxidation. The structure is
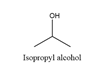
2-Methylpropanol will produce 2-methylpropanal on oxidation. The structure is
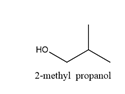
Cyclohexanol will produce cyclohexanone on oxidation and the structure is
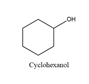
Explanation of Solution
The OH group is attached to the
Alcohols have -OH group. The hydrogen in this -OH is lost and it undergoes oxidation resulting in the formation of a carbonyl compound with oxygen double-bonded to carbon. This carbonyl center makes the carbonyl group polar.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





