
(a)
Interpretation:
The reagent and condition is to be proposed for step 1.
Concept introduction:
Friedal – Crafts alkylation (or acylation):
Friedal – Crafts alkylation (or acylation) is one of the electrophilic substitution reaction.
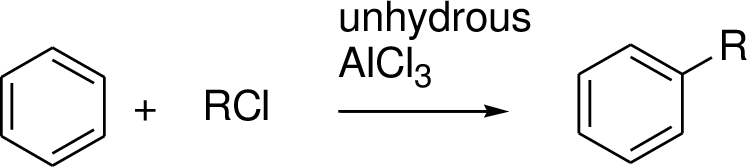
(b)
Interpretation:
The reagent and condition is to be proposed for step 2 and step 3.
Concept introduction:
Oxime formation:
(c)
Interpretation:
The reductive amination is to be explained and the explanation has to be given for two step synthesis of amination is used rather than reductive amination.
Concept introduction:
Reductive amination reaction: Amination is the process by which an
The conversion of Carbonyl group in to amine via imine intermediate is called reductive amination.
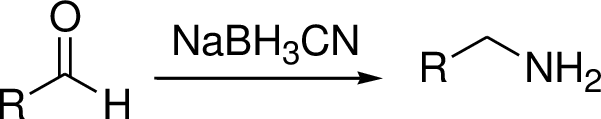
(D)
Interpretation:
The reagent and condition is to be proposed for step 4 and step 5.
Concept introduction:
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like LAH or NaBH4 which provides alcohol.
(E)
Interpretation:
The possible stereoisomer’s has to be shown if the product is chiral.
Concept introduction:
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Following is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forward(b) State the reagents needed to convert benzoic acid into the following compounds. (i) C6H§COCI (ii) C,H$CH2OH (iii) C6H$CONHCH3arrow_forward
- The following questions concern ethyl (2-oxocyclohexane)carboxylate.(a) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by a Dieckmann cyclization.(b) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by acylation of a ketone.(c) Write structural formulas for the two most stable enol forms of ethyl (2-oxocyclohexane)carboxylate.(d) Write the three most stable resonance contributors to the most stable enolate derived from ethyl (2-oxocyclohexane)carboxylate.(e) Show how you could use ethyl (2-oxocyclohexane)carboxylate to prepare 2-methylcyclohexanone.(f) Give the structure of the product formed on treatment of ethyl (2-oxocyclohexane)-carboxylate with acrolein (H2C=CHCH=O) in ethanol in the presence of sodium ethoxidearrow_forward(b) Propose a synthesis of (2,2-dimethylpropyl)benzene from benzene.arrow_forwardPropose single-step and multistep syntheses of acid derivatives from compoundscontaining other functional groups. Propose multistep syntheses using acid derivatives as starting materials and intermediates.arrow_forward
- (c)Show step by step how to synthesize methoxybenzene from benzene.arrow_forwardPredict the major organic product of each of the following reactions.arrow_forwardCompound A undergoes an acid-catalyzed hydrolysis. One of the products (B) that is isolated gives the following 1H NMR spectrum. Identify the compounds A and Carrow_forward
- How could you convert butanenitrile into the following compounds? Write each step showing the reagents needed. (a) 1-Butanol (b) Butylaminearrow_forwardBenzoic acid, Ph-COOH (C6H5CO2H), is not soluble in water while it dissolves in ether (diethyl ether), (CH3CH2)2O. Yet upon treatment with sodium hydroxide, benzoic acid turns hydrophilic and dissolves in water. Provide chemical explanation of this observation.arrow_forwardIsoerythrogenic acid, C18H26O2, is an acetylic fatty acid that turns a vivid blue on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acid, CH3(CH2)16CO2H, is produced. Ozonolysis of isoerythrogenic acid yields the following products: formaldehyde, CH2O, malonic acid, HO2CCH2CO2H, adipic acid, HO2C(CH2)4CO2H, and the aldehyde carboxylic acid, OHC(CH2)6CO2H. Provide a structure for isoerythrogenic acid.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


