
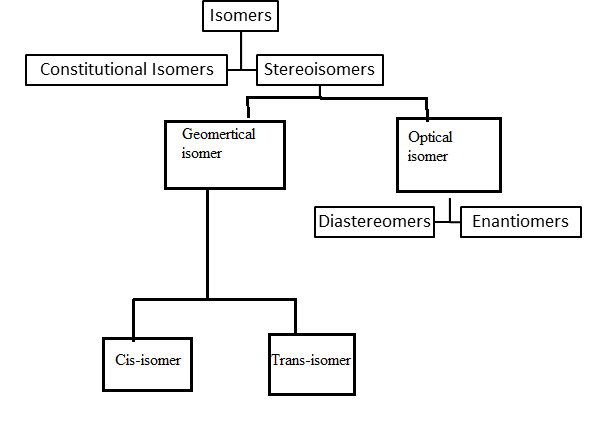
Interpretation: A concept map is to be drawn to show how the vocabulary words (Isomers, Constitutional isomers, Stereoisomers, Cis-trans isomers, and Enantiomers) are related.
Concept Introduction: Compounds in which molecular formulas are the same but chemical structures are different are known as isomers.
Answer to Problem 27LC
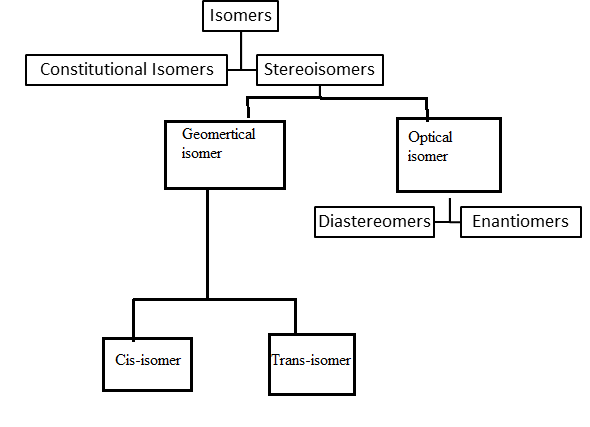
Explanation of Solution
Given Information: Isomers, Constitutional isomers, Stereoisomers, Cis-trans isomers, and Enantiomers are related to each other.
Compounds in which molecular formulas are the same but chemical structures are different are known as isomers. There are two types of isomers namely Constitutional isomers & Stereoisomers.
Compounds in which atoms are connected or bonded differently are called Constitutional isomers whereas compounds that differ due to orientation in space are called Stereoisomers.
Kind of Stereoisomers in which compounds are a mirror image of each other is known as Enantiomers. On the other hand, isomers that do not mirror images of each other are called Diastereomers.
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.arrow_forward(c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forward
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





