
(a)
Interpretation: The primary carbon needs to be identified.
Concept Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen
- Secondary carbon: When a carbon atom is attached to two carbon atoms and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(a)
Explanation of Solution
In the given molecule,
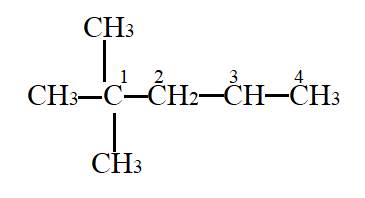
Carbon atom labelled as
(b)
Interpretation- To identify the secondary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(b)
Explanation of Solution
In the given molecule,
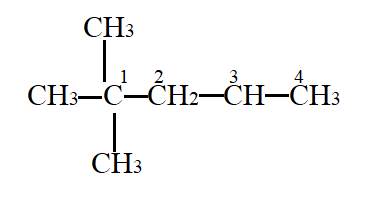
Carbon atom labelled as
(c)
Interpretation- To identify the tertiary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(c)
Explanation of Solution
In the given molecule,
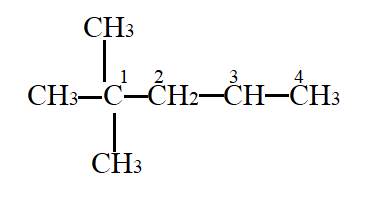
Carbon atom labelled as
(d)
Interpretation- To identify the quaternary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(d)
Explanation of Solution
In the given molecule,
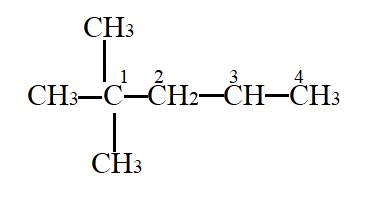
Carbon atom labelled as
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





