
(a)
Interpretation − To state the reason for incorrect name and write the correct names.
Concept Introduction − IUPAC naming follows rules as:
- The parent chain has highest number of carbon atoms.
- End part of name determines the number of carbon atoms in parent chain.
- Numbering is done in such a way that substituents get the lowest number.
- The leading part of name determine the type of substituents, the number determine the location of substituents and prefix determines the number of same substituents.
(a)
Explanation of Solution
The name of given compound is
The correct name will be
(b)
Interpretation − To state the reason for incorrect name and write the correct names.
Concept Introduction − IUPAC naming follows rules as:
- The parent chain has highest number of carbon atoms.
- End part of name determines the number of carbon atoms in parent chain.
- Numbering is done in such a way that substituents get the lowest number.
- The leading part of name determine the type of substituents, the number determine the location of substituents and prefix determines the number of same substituents.
(b)
Explanation of Solution
The name of given compound is pentane. According to given name, the parent chain has three carbon atoms. Two methyl substituents are present on first and third carbon atom.
But according to IUPAC rules, the parent chain must be longest, hence the methyl group present at the first and third carbon atom will be considered as a part of parent chain as shown in image.
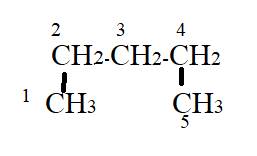
According to the structure the longest carbon will be of five carbon atoms with no substituents.
The correct name will be pentane.
(c)
Interpretation − To state the reason for incorrect name and write the correct names.
Concept Introduction − IUPAC naming follows rules as:
- The parent chain has highest number of carbon atoms.
- End part of name determines the number of carbon atoms in parent chain.
- Numbering is done in such a way that substituents get the lowest number.
- The leading part of name determine the type of substituents, the number determine the location of substituents and prefix determines the number of same substituents.
(c)
Explanation of Solution
The name of given compound is.
The numebering given to susbtituents is not lowest. Numbering will b done as shown:
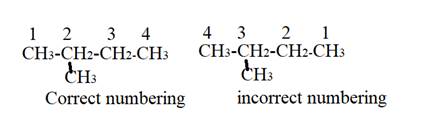
The lowest position that methyl substituent can get is at second carbon atom of parent chain.
Hence, the correct name will be
(d)
Interpretation − To state the reason for incorrect name and write the correct names.
Concept Introduction − IUPAC naming follows rules as:
- The parent chain has highest number of carbon atoms.
- End part of name determines the number of carbon atoms in parent chain.
- Numbering is done in such a way that substituents get the lowest number.
- The leading part of name determine the type of substituents, the number determine the location of substituents and prefix determines the number of same substituents.
(d)
Explanation of Solution
The name of given compound is.
From the given name, longest carbon chain has four carbon atoms and methyl groups are present third and fourth carbon atom of parent chain.
According to IUPAC rules, the parent chain must be longest carbon chain. Hence the methyl group present on fourth carbon will be considered as a part of longest carbon chain or parent chain shown as follows:
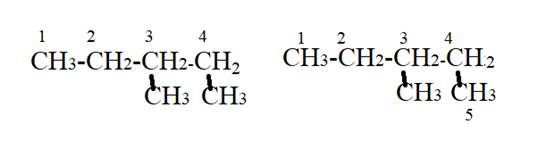
The parent chain will have five carbon atoms and methyl group is present on third carbon atom.
Hence, the correct name will be
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





