
Chemistry 2012 Student Edition (hard Cover) Grade 11
12th Edition
ISBN: 9780132525763
Author: Prentice Hall
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Question
Chapter 22, Problem 15STP
Interpretation Introduction
Interpretation: To identify the molecular structure of cis isomer.
Concept Introduction: Stereoisomers are molecules that have the same number of carbon atoms but differ in the arrangement of atoms.
The two categories of stereoisomers are as follows:
- Cis-trans isomers
- Enantiomers
Expert Solution & Answer
Answer to Problem 15STP
The molecular structure that represents a cis isomer is (C).
Explanation of Solution
The geometric isomers in which the atoms are joined in the same order are known as cis isomers while the isomers in which the arrangement of atoms is in a different order or on the opposites of the double bond are known as trans isomers.
The given molecular structure of the cis isomer is represented as follows:
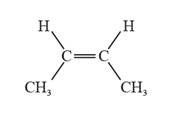
Conclusion
Thus, the molecular structure that represents a cis isomer is (C).
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
Ch. 22.1 - Prob. 1SPCh. 22.1 - Prob. 2SPCh. 22.1 - Prob. 3SPCh. 22.1 - Prob. 4SPCh. 22.1 - Prob. 5SPCh. 22.1 - Prob. 6SPCh. 22.1 - Prob. 7LCCh. 22.1 - Prob. 8LCCh. 22.1 - Prob. 9LCCh. 22.1 - Prob. 10LC
Ch. 22.1 - Prob. 11LCCh. 22.1 - Prob. 12LCCh. 22.1 - Prob. 13LCCh. 22.2 - Prob. 14LCCh. 22.2 - Prob. 15LCCh. 22.2 - Prob. 16LCCh. 22.2 - Prob. 17LCCh. 22.2 - Prob. 18LCCh. 22.3 - Prob. 19SPCh. 22.3 - Prob. 20SPCh. 22.3 - Prob. 21LCCh. 22.3 - Prob. 22LCCh. 22.3 - Prob. 23LCCh. 22.3 - Prob. 24LCCh. 22.3 - Prob. 25LCCh. 22.3 - Prob. 26LCCh. 22.3 - Prob. 27LCCh. 22.4 - Prob. 28LCCh. 22.4 - Prob. 29LCCh. 22.4 - Prob. 30LCCh. 22.4 - Prob. 31LCCh. 22.4 - Prob. 32LCCh. 22.5 - Prob. 33LCCh. 22.5 - Prob. 34LCCh. 22.5 - Prob. 35LCCh. 22.5 - Prob. 36LCCh. 22.5 - Prob. 37LCCh. 22.5 - Prob. 38LCCh. 22.5 - Prob. 39LCCh. 22.5 - Prob. 40LCCh. 22 - Prob. 41ACh. 22 - Prob. 42ACh. 22 - Prob. 43ACh. 22 - Prob. 44ACh. 22 - Prob. 45ACh. 22 - Prob. 46ACh. 22 - Prob. 47ACh. 22 - Prob. 48ACh. 22 - Prob. 49ACh. 22 - Prob. 50ACh. 22 - Prob. 51ACh. 22 - Prob. 52ACh. 22 - Prob. 53ACh. 22 - Prob. 54ACh. 22 - Prob. 55ACh. 22 - Prob. 56ACh. 22 - Prob. 57ACh. 22 - Prob. 58ACh. 22 - Prob. 59ACh. 22 - Prob. 60ACh. 22 - Prob. 61ACh. 22 - Prob. 62ACh. 22 - Prob. 63ACh. 22 - Prob. 64ACh. 22 - Prob. 65ACh. 22 - Prob. 66ACh. 22 - Prob. 67ACh. 22 - Prob. 68ACh. 22 - Prob. 69ACh. 22 - Prob. 70ACh. 22 - Prob. 71ACh. 22 - Prob. 72ACh. 22 - Prob. 73ACh. 22 - Prob. 74ACh. 22 - Prob. 75ACh. 22 - Prob. 76ACh. 22 - Prob. 77ACh. 22 - Prob. 78ACh. 22 - Prob. 79ACh. 22 - Prob. 80ACh. 22 - Prob. 81ACh. 22 - Prob. 82ACh. 22 - Prob. 84ACh. 22 - Prob. 85ACh. 22 - Prob. 86ACh. 22 - Prob. 87ACh. 22 - Prob. 89ACh. 22 - Prob. 90ACh. 22 - Prob. 91ACh. 22 - Prob. 92ACh. 22 - Prob. 93ACh. 22 - Prob. 94ACh. 22 - Prob. 95ACh. 22 - Prob. 96ACh. 22 - Prob. 97ACh. 22 - Prob. 98ACh. 22 - Prob. 99ACh. 22 - Prob. 100ACh. 22 - Prob. 101ACh. 22 - Prob. 102ACh. 22 - Prob. 103ACh. 22 - Prob. 104ACh. 22 - Prob. 105ACh. 22 - Prob. 106ACh. 22 - Prob. 107ACh. 22 - Prob. 108ACh. 22 - Prob. 109ACh. 22 - Prob. 110ACh. 22 - Prob. 111ACh. 22 - Prob. 1STPCh. 22 - Prob. 2STPCh. 22 - Prob. 3STPCh. 22 - Prob. 4STPCh. 22 - Prob. 5STPCh. 22 - Prob. 6STPCh. 22 - Prob. 7STPCh. 22 - Prob. 8STPCh. 22 - Prob. 9STPCh. 22 - Prob. 10STPCh. 22 - Prob. 11STPCh. 22 - Prob. 12STPCh. 22 - Prob. 13STPCh. 22 - Prob. 14STPCh. 22 - Prob. 15STPCh. 22 - Prob. 16STP
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY