
(a)
Interpretation- To name the given compound.
Introduction-
- Identify the longest parent chain.
- Identify the substituents.
- In
alkenes , the lowest number is given to double bonds rather than substituents. - The position of the double bond is mentioned while naming.
- If the substituents are more than one, prefixes like di, tri, tetra, etc., are used.
- The name of the compound starts with substituents with their position in the parent chain. They are written in alphabetical order.
- Numbers and names are separated by dash while a number are separated by commas.
- The chain name is based on the number of carbon atoms present like meth, eth, prop, but, pent, hex, hept, oct, non, dec,
- Suffix “ene” is used for the
alkane parent chain and “yl is used for substituents. - Alkenes show cis-trans isomerism. If the same groups are present on the same side of the double bond, it is “cis”. If the same groups are present on the opposite side of the double bond, it is “trans”.
(a)
Answer to Problem 46A
The name of the compound is
Explanation of Solution
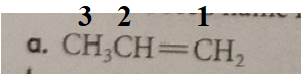
The compound has the longest parent chain with three carbon atoms. Hence the parent name chain will be prop with suffix “ene”.
Therefore, the name of the compound is
Therefore, the name of the compound is
(b)
Interpretation- To name the given compound.
Introduction- IUPAC nomenclature follows some rules for naming the compounds:
- Identify the longest parent chain.
- Identify the substituents.
- In alkenes, the lowest number is given to double bonds rather than substituents.
- The position of the double bond is mentioned while naming.
- If the substituents are more than one, prefixes like di, tri, tetra, etc., are used.
- The name of the compound starts with substituents with their position in the parent chain. They are written in alphabetical order.
- Numbers and names are separated by dash while a number are separated by commas.
- The chain name is based on the number of carbon atoms present like meth, eth, prop, but, pent, hex, hept, oct, non, dec,…
- Suffix “ene” is used for the alkane parent chain and “yl is used for substituents.
- Alkenes show cis-trans isomerism. If the same groups are present on the same side of the double bond, it is “cis”. If the same groups are present on the opposite side of the double bond, it is “trans”.
(b)
Answer to Problem 46A
The name of the compound is
Explanation of Solution
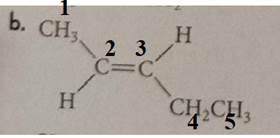
The compound has the longest parent chain with five carbon atoms. Hence, the parent name chain will be pent with the suffix “ene”. The double bond is present at the second carbon.
Same group, hydrogen is present on the opposite side of the double bond; hence, it shows a trans isomer.
Therefore, the name of the compound is
Therefore, the name of the compound is
(c)
Interpretation- To name the given compound.
Introduction- IUPAC nomenclature follows some rules for naming the compounds:
- Identify the longest parent chain.
- Identify the substituents.
- In alkenes, the lowest number is given to double bonds rather than substituents.
- The position of the double bond is mentioned while naming.
- If the substituents are more than one, prefixes like di, tri, tetra, etc., are used.
- The name of the compound starts with substituents with their position in the parent chain. They are written in alphabetical order.
- Numbers and names are separated by dash while a number are separated by commas.
- The chain name is based on the number of carbon atoms present like meth, eth, prop, but, pent, hex, hept, oct, non, dec,…
- Suffix “ene” is used for the alkane parent chain and “yl is used for substituents.
- Alkenes show cis-trans isomerism. If the same groups are present on the same side of the double bond, it is “cis”. If the same groups are present on the opposite side of the double bond, it is “trans”.
(c)
Answer to Problem 46A
The name of the compound is
Explanation of Solution
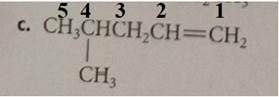
The compound has the longest parent chain with five carbon atoms. Hence the parent name chain will be pent with the suffix “ene”. The double bond is present at first carbon.
Substituent has one carbon atom, and it is present on the fourth carbon atom of the parent chain.
Therefore, the name of the compound is
Therefore, the name of the compound is
(d)
Interpretation- To name the given compound.
Introduction- IUPAC nomenclature follows some rules for naming the compounds:
- Identify the longest parent chain.
- Identify the substituents.
- In alkenes, the lowest number is given to double bonds rather than substituents.
- The position of the double bond is mentioned while naming.
- If the substituents are more than one, prefixes like di, tri, tetra, etc are used.
- The name of the compound starts with substituents with their position in the parent chain. They are written in alphabetical order.
- Numbers and names are separated by dash while a number are separated by commas.
- The chain name is based on the number of carbon atoms present like meth, eth, prop, but, pent, hex, hept, oct, non, dec,…
- Suffix “ene” is used for the alkane parent chain and “yl is used for substituents.
- Alkenes show cis-trans isomerism. If the same groups are present on the same side of the double bond, it is “cis”. If the same groups are present on the opposite side of the double bond, it is “trans”.
(d)
Answer to Problem 46A
The name of the compound is
Explanation of Solution
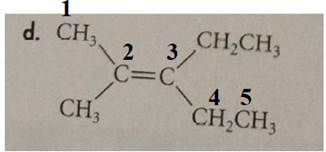
The compound has the longest parent chain with five carbon atoms. Hence the parent name chain will be pent with the suffix “ene”. The double bond is present at the second carbon.
Methyl substituent is present at the second carbon and ethyl substituent is present at the third carbon.
Methyl substituents are present on the same side of the double bond. Hence this is a cis isomer.
Therefore, the name of the compound is
Therefore, the name of the compound is
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





