
Concept explainers
a.
Interpretation:
From a given pair of compound, which one is higher boiling point has to be determined.
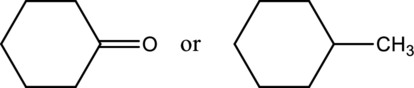
Concept introduction:
Boiling point of aldehyde, ketone, alcohol and hydrocarbons:
Carbonyl compounds such as
b.
Interpretation:
From a given pair of compound, which one is higher boiling point has to be determined.
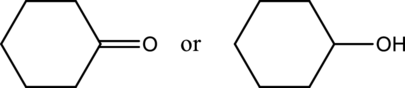
Concept introduction:
Refer to part a.
c.
Interpretation:
From a given pair of compound, which one is higher boiling point has to be determined.
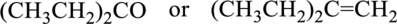
Concept introduction:
Refer to part a.
d.
Interpretation:
From a given pair of compound, which one is higher boiling point has to be determined.
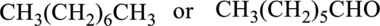
Concept introduction:
Refer to part a.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Principles of General, Organic, Biological Chemistry
- Give the IUPAC name for each compound.arrow_forwardWhich compound in each pair has the higher boiling point? Explainarrow_forwardGive the IUPAC name for each compound. OH a. CH3CH(CH₂)4CH3 (select) OH (CH3CH₂)2CHCHCH₂CH3 (select) b. C. d. CH3 OH (select) (select) (select) OH (select) (select) (select)arrow_forward
- Explain why diethyl ether (CH3CH2OCH2CH3) and butan-1-ol (CH3CH2CH2CH2OH) have similar solubility properties in water, but butan-1-ol has a much higher boiling point.arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent.a. HClb. CH3COClc. (CH3CO)2Od. excess CH3Ie. (CH3)2C = Of. CH3COCl, AlCl3g. CH3CO2Hh. NaNO2, HCli. Part (b), then CH3COCl, AlCl3j. CH3CHO, NaBH3CNarrow_forward33. What is each compound's systematic name? a. CH₂C=CCH₂CHCH, b. CH₂C=CCH₂CHCH₂ Br CH₂CH₂CH₂ CH₁ c. CH₂C=CCH₂CCH, d. CH₂CHCH₂C=CCHCH CH, CH,arrow_forward
- Give the IUPAC name for each compound.arrow_forwardArrange the following compounds in order of increasing boiling point.Explain. (CH3)2CHCH2OH (CH3)2CH(CH2)2CH2OH (CH3)2CH(CH2)3CH2OHarrow_forwardDraw the products formed when each alcohol is oxidized with K 2Cr 2O 7. In some cases, no reaction occurs.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
