
Concept explainers
(a)
Interpretation: The Newman projections of
Concept introduction: Various interconvertible forms that result from rotation around the
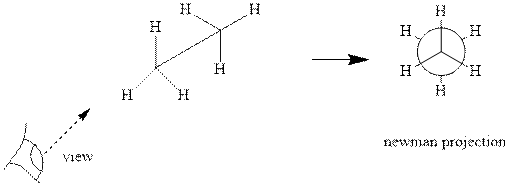
As rotation is carried out along
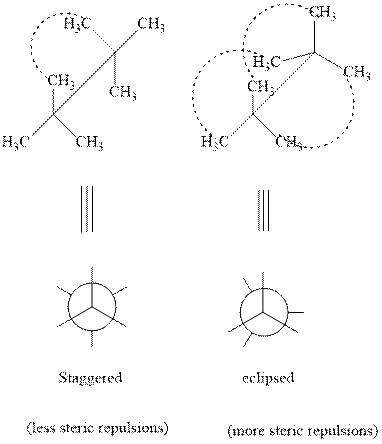
(b)
Interpretation: The indicated molecules should be drawn in chair conformations.
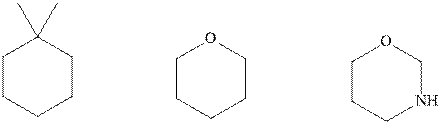
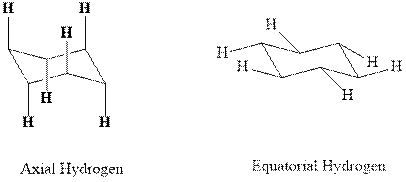
Concept introduction:In chair form, all the
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: Structure and Function
- Draw the structures of 1,3-pentadiene and 1,4-pentadiene and label the carbons. Predict the trendin C-C single bond lengths in the two compounds.arrow_forward(a) What is the IUPAC name for the following molecule (including absolute configurations if required)? (b) Draw the Newman projections looking down the C3-C4 bond that represent all the three staggered conformations for the molecule shown above. (c) Given that the strain energies below, calculate the strain energies for each conformer and identify which is the most stable. Gauche interaction Strain Energy HOCH3 0.0 kJ mol- HOCH;CH; |0.4 kJ mol- 3.8 kJ mol- | 4.3 kJ mol- CH;CH3 CH;CH2CH3 |CH2CH; CH2CH; 4.6 kJ mol- Newman Projection of Conformer 1 Strain Energy of Conformer 1 Newman Projection of Conformer 2 Strain Energy of Conformer 2 Newman Projection of Conformer 3 Strain Energy of Conformer 3arrow_forwardDraw a dash-wedge structure for (1R)-1-bromo-1,3,3-trimethylcyclohexane.arrow_forward
- Following is the Structural formal of Chloropropane H H H 2. H Draw Newman projections for all staggered and eclipsed conformation formed by rotation about C1 - C2 bond from 0° to 360°. b) Which Staggered conformation(s) has the lowest energy; which has the highest energy? c) Which eclipsed conformation(s) has the lowest energy, which has thhe highest energy?arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forward-1. What is the equivalent bond-line formula for the molecule, HOCH-CH(CH-CH₂)-CH(CH)? HO. (B) HO (C) HO. -2. What is the equivalent structure for this molecule using a hond-line formula? A) C) Br Br (D) (B) HO (CH₂)2CH(CH₂) CHBICHCH, Br CH₂CH3 CH3 yazparrow_forward
- Sighting along the appropriate C-C bond of neopentane C(CH3)4: a) Draw its Newman projections. b) How much energy does the most unstable conformation possess? c) Make the energy diagram for the different conformers.arrow_forward(5) Draw the most stable chair conformation of the cyclohexane shown below. CH3 CI H3C Br OH CH3arrow_forwardQ2. Which one of the following structures is the most stable conformation for 2-bromo butane (viewing down the C2-C3 bond axis where C2 is front carbon and C3 is back carbon) Br H H H **** CH, CH₂ (B) Br (A) H H (D)arrow_forward
- 13a. Draw the correct skeletal structure of 5-methyl-1-hexanol. provided (13b.) draw in skeletal structure its most stable conformer looking along C3-C4 (* hydrogens that are directly connected to the Newman projection ( and uii the Newman Projection ;; Draw the 13a. 13b. draw the correct skeletal structure here draw the correct skeletal structure here 4arrow_forward6. Newman projections to Lewis structures From the following Newman projections, draw the corresponding Lewis structure in the same conformation. A) B) H. H CH3 H₂C N H OH CI Br CH3 H OH CH3arrow_forward(c)Conformational isomer is the different spatial arrangement of the atoms that resulted from rotation about a single bond. (i) Differentiate anti conformers and gauche conformers (ii) Using a newman projection, draw the anti conformer and gauche conformers of 3,3-dimethylheptane (viewed along the C3-C4 bond)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





