
(a)
Interpretation: The mechanism for monochlorination with the help of iodobenzene dichloride should be proposed.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
The mechanism for monochlorination comprises of three stages illustrated as follows:
Step1- Initiation via homolytic cleavage of
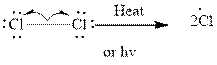
Step2-: Propagation: In first of the propagation steps,
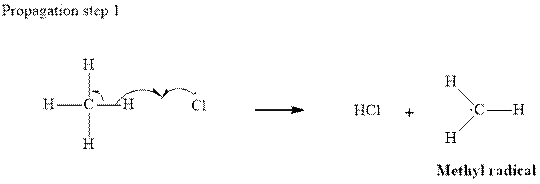
In subsequent propagation step chloromethyl radical abstracts
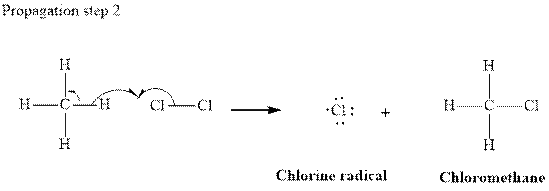
Step3: Termination: Radicals generated in propagation steps get quenched upon combination with one another. Thus termination steps are essentially the radical− radical combination illustrated as follows:
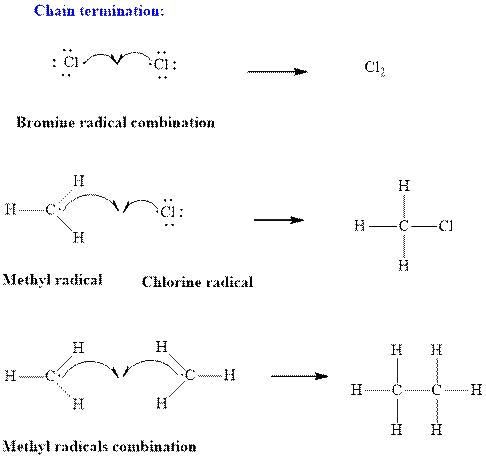
(b)
Interpretation: The three major sites of monochlorination present over steroidsshould be predicted.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
The mechanism for monochlorination comprises of three stages illustrated as follows:
Initiation via homolytic cleavage of
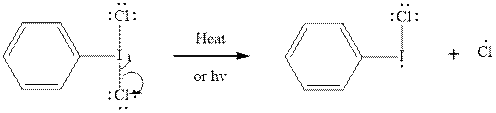
Step2-: Propagation: In first of the propagation steps

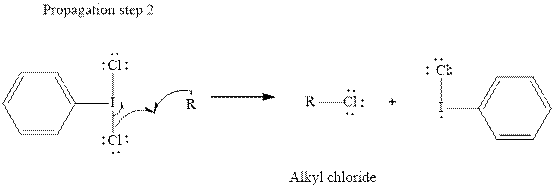
In subsequent propagation step alkyl radical abstracts
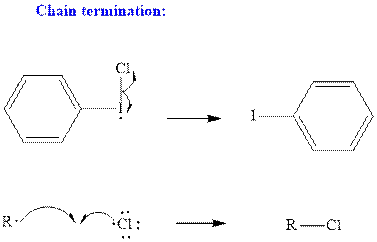
Step3: Termination: Radicals generated in propagation steps get quenched upon combination with one another. Thus termination steps are essentially the radical− radical combination illustrated as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: Structure and Function
- Alkenes undergo an addition reaction with borane in tetrahydrofuran (THF). For the reaction below: BH3 THF (solvent) a Draw the structure of the major organic product. Use the wedge/hash bond tools to indicate stereochemistry where it exists. Use wedge and hash bonds ONLY when needed to show reaction stereochemistry. If the reaction produces a racemic mixture, just draw one stereoisomer. opy aste ChemDoodlearrow_forwardAn alkene having the molecular formula C5H10 is treated sequentially with ozone (O3) and zinc/acetic acid to give the product/s shown. H3C O CH3CHCH + H. Draw a structural formula for the alkene. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. In cases where there is more than one answer, just draw one. P opy aste [* Previous Nextarrow_forward09:03 4G What is the correct IUPAC name for the following compound? .F "O. O A. 1-Fluoro-2,4-dinitrobenzene O B. Dinitrofluorobenzene O C. 1-Fluoro-4,6-dinitrobenzene O D. 1,3-Dinitr-1-fluorobenzene Add a caption... > Status (Custom) Z=Oarrow_forward
- Alkoxides R-O- can be used to synthesize alkenes as exemplified by the reaction shov below, where the symbol (et) indicates that the substance is dissolved in ethanol (CH;CH,OH) used as solvent: Br (1) + HO Br(et) (et) + (et) CH,CH,OH t-but-Br Ethoxide Isobutylene 9. The table to the right shows the results for the initial rate of this reaction when performed under different conditions (four different trials) at 25 °C. What is the rate law for this reaction? a) Rate = k [t-but-Br] b) Rate = k [t-but-Br] [ethoxide] c) Rate = k [t-but-Br]? d) Rate = k [ethoxide]? Trial Initial Initial Initial Rate (M/s) [t-but-Br] [Ethoxide] [isobutylene] 1 0.08 M 0.04 M 6.8 x 10-8 13.6 x 10-8 6.8 x 10-8 13.6 х 10-8 2 0.16 M 0.04 M 3 0.08 M 0.08 M 4 0.16 M 0.08 Marrow_forwardBelow is a structural formula. What is the corresponding condensed structural H formula? H1C1I Н Н OCH3NHC(CH3)2 H-CN-C-C CH3NHCH(CH3)2 OCH3NC(CH3)2 :Z―I OCH3NCH(CH3)2 H H H I— H -C-H H (arrow_forwardThe correct IUPAC name for this compound is : H₂C. H&G HyG CH₂ O a. (E)-3-methyl-4-isopropyl-3-hexene O b. (Z)-3-methyl-4-isopropyl-3-hexene O c. (Z)-3-ethyl-2,4-dimethyl-3-hexene O d. (Z)-2,3-dimethyl-2-isopropyl-2-pentene e. (E)-3-ethyl-2,4-dimethyl-3-hexenearrow_forward
- The compound below is treated with chlorine in the presence of light. CH3 CH3 CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Sn [F ?arrow_forwardEstimate the heat released when ethene(CH2=CH2) reacts with HBr to giveCH3CH2Br. Bond enthalpies areC-H : 412 kJ/mol; C-C : 348 kJ/mol;C=C : 612 kJ/mol; C-Br : 276 kJ/mol;Br-Br : 193 kJ/mol; H-Br : 366 kJ/mol. Choose the correct answer:1. 1036 kJ/mol2. 58 kJ/mol3. 424 kJ/mol4. 200 kJ/mol5. 470 kJ/molarrow_forwardBelow, write the names of 7 organic compounds whose open structures are given, and the open structures of 8 organic compounds that have been named according to the IUPAC naming system. (Consider also the case of R, S or E, Z. 5,5-bis (1,2-dimethylpropyl) nonane Cis-1,3-diisopropylcyclopentane 5-hydroxyhexanoic acid Bicyclo [2.2.1] heptane (S) -2-bromo-3-methylbutane Benzyl trans-3-phenyl-2-propenoate 1- (2-naphthyl) -6-phenyl-2,5-hexadione 1,1-dimethyl-2-cyclohexenolarrow_forward
- Suppose the alkene in the drawing area below is put in strong acid solution, for example a solution of HBr. Highlight each carbon that might become protonated to form a carbocation intermediate. (The carbocation intermediate might then react with something else to form a final product.) سعدarrow_forwardIn light of the nitrogen rule mentioned in Problem 12-17, what is the molecular formula of pyridine, M+=79?arrow_forwardCarbon–carbon bond dissociation enthalpies have been measured for many alkanes. Identify the alkane in each of the following pairs that has the lower carbon–carbon bond-dissociation enthalpy, and explain the reason for your choice. (a) Ethane or propane (b) Propane or 2-methylpropane (c) 2-Methylpropane or 2,2-dimethylpropane (d) Cyclobutane or cyclopentanearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

