
Concept explainers
(a)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
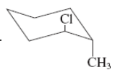
(b)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be calculated.
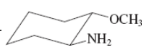
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(c)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
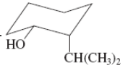
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(d)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
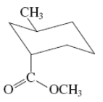
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(e)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
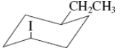
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(f)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
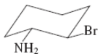
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(g)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
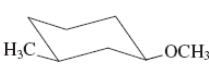
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(h)
Interpretation:The value of free energy for ring flip to other conformations of the molecule needs to be determined.
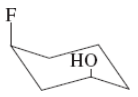
strong>Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(i)
Interpretation:Thevalue of free energy for ring flip to other conformations of the molecule needs to be determined.
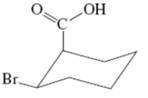
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
(j)
Interpretation:Thevalue of free energy for ring flip to other conformations of the molecule needs to be determined.
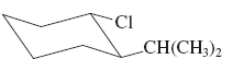
Concept Introduction: The free energy from equatorial to axial conversion is negative and from equatorial to axial is positive.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: Structure and Function
- Connections to biology 6. Idose is a monosaccharide (i.e. sugar) molecule. The structure shown on the left below represents one form of idose in a six-membered ring. Draw the two chair conformations of this molecule using the templates. Note that the positions of carbon 1 are marked in all structures. OH HO 2 OH LO 1, OH OH OHarrow_forwardDraw a stereoisomer of cis-1,2-dimethylcyclohexane. (Note that the question asks for a different stereoisomer of the named compound and not the named compound itself.) • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • In cases where there is more than one answer, just draw one. Y TAYY n[] ChemDoodlearrow_forwardreq Zreq 2req 2req 2req [Review Topics) [References] Cyclohexane derivatives exist primarily in the most stable of the available chair conformations. Give the position, axial or equatorial, of each of the three groups shown when the ring is in the most stable chair conformation. If a group divides its time equally between axial and equatorial positions, indicate this with ax/eq. The table of "Axial Strain Energies for Monosubstituted Cyclohexanes" found in the "Strain Energy Increments" section of the Reference tool is useful for answering this question. OH HC Group a is Group bis Group cis Submit Answer OH b Retry Entire Group 9 more group attempts remaining o O · 9020 0arrow_forward
- 1 of 2 2. Draw the two chair conformations of cis-1,3-dimethylcyclohexane. Circle the conformation that is favored (most stable). The, using the values given below, calculate (show your work) a) the AG between the chair conformers, and b) the equilibrium constant at room temperature. (AG = -RTlnKeg) R = 8.314 J/mol•K 1,3-diaxial interaction Strain energy (kJ/mol) Me/H Me/Me 3.8 15.5arrow_forwardWhat is the total strain energy of 2,2-dimethylpropane in an eclipsed conformation? Given: For CH3 eclipsed to H (both attached to adjacent C's), the total strain energy is 6 kJ/mol. Select one: A. 12 kJ В. 18 kJ С. 21 kJ D. 11.4 kjarrow_forward2 2. Draw all possible conformations for 1,1,2-tribromopropane. Label all unfavc interactions. Which conformation is most stable? Explain. TIarrow_forward
- By considering viewed through the C-2-C-3 bond, which below conformations of 2,3- dibromobutane show the most stable and the least stable conformation? Clearly explain the reason, why? S CH3 H H Br. H3C, Br H3C Br Br Br H. Br CH3 ČH3 H I II III Br Br H3C, Br H3C H. H3C" H. Br ČH3 IV Varrow_forwardDraw the structure of cis-1,2-dimethylcyclohexane. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • You do not have to explicitly draw H atoms. opy astearrow_forwardThe dehydrogenation of butane to trans-but-2-ene has ∆H° = +116 kJ>mol (+27.6 kcal> mol) and ∆S° = +117 J>kelvin@mol (+28.0 cal>kelvin@mol).(a) Compute the value of ∆G° for dehydrogenation at room temperature (25 °C or 298 °K). Is dehydrogenation favored or disfavored?(b) Compute the value of ∆G for dehydrogenation at 1000 °C, assuming that ∆S and ∆H are constant. Is dehydrogenation favored or disfavored?arrow_forward
- Draw the most stable AND most unstable conformations of the following molecules. Briefly explain your answer. Note, for linear structures looking at rotation around the bond depicted in red. HO OH HN Brarrow_forwardRank the conformations of n-butane with reference to its potential energy from the most stable to the least stable. Rank from the most stable to the least stable.To rank items as equivalent, overlap them.arrow_forwardDraw the structure of the organic product of the reaction between cyclohexene and OsO4, H₂O2. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. Separate multiple products using the + sign from the drop-down menu. • In cases where there is more than one answer, just draw one. ● MATIL ? Ⓡ ChemDoodle Sn [Farrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





