
Concept explainers
Interpretation: Different structures for formula C5H10with one ring should be determined.
Concept introduction: C5H10have two hydrogen atoms less than the molecular formula of C5H12, i.e. it contains either a double bond or a cyclic group. So in functional groups,
Answer to Problem 21P
- Cyclopentane
- Methylcyclobutane
- 1,1-dimethyl cyclopropane
- 1,2- dimethyl cyclopropane
- Ethylcyclopropane
Explanation of Solution
Considering the structural formula C5H10 with a ring, the maximum number of carbon in that ring can be equal to or less than 5.
So the structures will be:
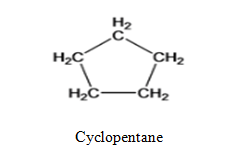
Cyclopentane
Other structures are:
Cyclobutane with one methyl substituent which is named as Methylcyclobutane
(According to IUPAC)
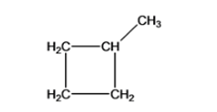
Cyclopropane with 2 methyl functional group (1, 1-dimethyl cyclopropane )
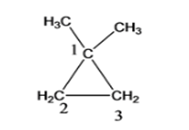
Cyclopropane with 2 methyl functional group, where functional group is attached to the adjacent C atoms of the cyclic groups (1,2- dimethylcyclopropane)
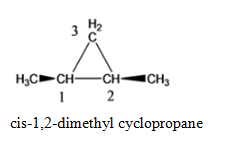
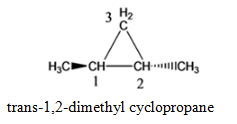
cis-1,2-dimethyl cyclopropane trans-1,2-dimethyl cyclopropane
Cyclopropane is another structure with 1 ethyl (CH2CH3) functional group is named as Ethylcyclopropane
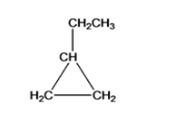
The five different structures can be written for the structural equation C5H10 and they are Cyclopentane, Methylcyclobutane, 1,1-dimethylcyclopropane, 1,2-dimethyl cyclopropane, ethylcyclopropane.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Structure and Function
- Your roommate, a chemistry major, claims to have synthesized the compound CH5 in the lab. Why is that not possible?arrow_forwardThe formula CH3C=CCH3 represents an alkyne. O an alkane. an aromatic compound. O a cycloalkane. O an alkene.arrow_forwardОН SH ÇH5 CH, O CH2 O || НN—CH—C—NH—СH—С-NH—СH-С-NH—СH—С—NH—СH -С—NH—CH-С—0 CH2 O || CH3 O H но LH-L "Î. Part A Write the abbreviated versions of the following structural formula usingthe three-letter abbreviations. Spell out the full name of the compound. Submit Request Answer Part B Write the abbreviated versions of the following structural formula using the one-letter abbreviations. Spell out the full name of the compound. Submit Request Answerarrow_forward
- Organic compounds that contain double or triple carbon- carbon bonds are called ____ compounds. The name of the alkene with the formula CH3-CH=CH-CH2 -CH3 is ____. Organic compounds that contain the benzene ring are called ____compounds.arrow_forwardHow do Substituted polyethylenes make up an entire class of polymers? Explain with an example?arrow_forwardDecane has the structure: CH3(CH2)9CH3. What does the prefix "dec-" specifically indicate about the structure of this compound? Multiple Choice It contains a straight chain of carbons. It contains 10 carbons in a continuous chain. It is an alkane. It is a saturated hydrocarbon.arrow_forward
- Q1: Draw all of the structural isomers possible for the alkane with the molecular formula CeH14, and write the naming of all compounds.arrow_forwardThe heats of combustion of methane and butane are 890 kJ/mol (212.8 kcal/mol) and 2876 kJ/mol (687.4 kcal/mol), respectively. When used as a fuel, would methane or butane generate more heat for the same mass of gas? Which would generate more heat for the same volume of gas?arrow_forwardYou are teaching a class in organic chemistry to grade 12 students. Outline the differences in 3 physical properties between alkanes, alcohols, and carboxylic acids. Note: they all have the same hydrocarbon length.arrow_forward
- Gasohol is a mixture of 90% gasoline and 10% ethanol, CH 3CH 2OH. Ethanol is considered an environmentally friendly fuel additive because it can be made from a renewable source—sugarcane. Ethanol burns in air to form CO 2 and H 2O, and, like the combustion of alkanes, this reaction also releases a great deal of energy. Write a balanced equation for the combustion of ethanol.arrow_forwardDraw the skeletal ("line") structure of 3-chloro-2-methyloctane. Click and drag to start drawing a structure.arrow_forwardIs there any difference between 2-methylpropane and isobutane? Why is the structure in the image not named 2-methylpropane but isobutane. What is meant by "iso"?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




