
Concept explainers
Interpretation:Potential-energy diagram for chair−chair interconversion of methylcyclohexane should be sketched.
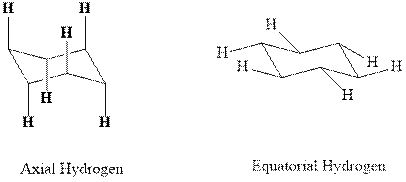
Concept introduction:In chair form all the
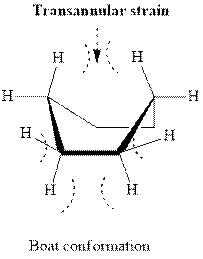
In boat type conformation, flagpole interaction is found as illustrated below.
Usually, the stable conformations have bulkier groups such as isopropyl,
Trending nowThis is a popular solution!

Chapter 4 Solutions
Organic Chemistry: Structure and Function
- From the data in Figure 4-12 and Table 4-1, estimate the percentages of molecules that have their substituents in an axial orientation for the following compounds: (a) Isopropylcyclohexane (b) Fluorocyclohexane (c) Cyclohexanecarbonitrile, C6H11CNarrow_forwardLook at Figure 4-12 on page 105, and estimate the percentages of axial and equatorial conformations present at equilibrium in bromocyclohexane.arrow_forwardBelow are isomers of tert-butylcyclohexanol in conformational structures. Two are cis-2-tert- butylcyclohexanol and two are cis-3-tert-butylcyclohexanol. Indicate which is the more stable conformation for each pair, and indicate which of all four conformations is the most stable. A OH Ex HO B D OH OHarrow_forward
- Identify the correct chair conformations of the following compound and then indicate which one is more stable. OH OH OH HO 1 || 4 OI and II are correct chair structures and I is most stable OI and II are correct chair structures and II is most stable OI and III are correct chair structures and III is most stable O II and III are correct chair structures and II is most stable O II and III are correct chair structures and III is most stable |||arrow_forwardIn the lowest energy chair conformation of cis-1,3-dimethylcyclohexane, how many axial positions are occupied by hydrogen atoms? * O 2arrow_forwardThe most stable chair conformation of 4-ethyl-1-isopropyl-2-methyl-cyclohexane where the isopropyl group is cis to the ethyl group and trans to the methyl group.arrow_forward
- 11. s) Draw the most stable conformation of 1,3-dichloro-2,4-difluorocyclohexane: 12. Which of the following represents the most stable conformation of 1,2,4,5- tetramethyleyclohexane? (one correct answer) CH b) CH HC H- H- CH HC CH, CH 6. Give IUPAC name for the following molecule. Include "cis" and "trans" designations if applicable. H2C-CH3 H -CH2 H- H Brarrow_forward5. Draw the two chair conformations ("ring filips") of trans –1- ethyl-4-methylcyclohexane to show the axial and equatorial substituents (including all hydrogens). Then determine which of the two conformations is more stable and why.arrow_forwardComplete the given chair conformation of cyclohexane by adding the listed substituents to the specified position and bond direction. Substituent Position Bond direction CH3 CH3 OH F 3 6 1 3 axial axial equatorial equatorialarrow_forward
- Given cyclohexane in a chair conformation, construct the more stable conformation of cis‑1‑methyl‑2‑propylcyclohexane by filling in the missing atoms or groups. Use the numbering provided on the ring.arrow_forward4.(a). Draw the structures of (i) cis-1,4-Dibromocyclohexane and (ii) trans-1,4- Dibromocyclohexane. 4(b). Draw the most stable conformers of (i) cis-1,4-Dibromocyclohexane and (ii) trans-1,4-Dibromocyclohexane. 4(c)Which is more stable? (i) cis-1,4-Dibromocyclohexane or (ii) trans-1,4- Dibromolcyclohexane. Explain the reason for your choice.arrow_forwardDefine cyclohexane chair conformation ?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



