
Concept explainers
(a)
Interpretation:Most stable conformation for indicated substituted cyclohexane should be identified.
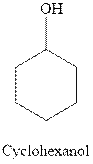
Concept introduction:In chair form, all the
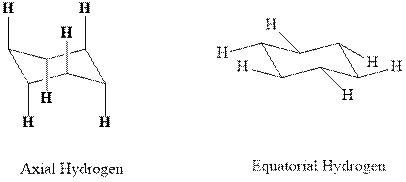
(b)
Interpretation: Most stable conformation for indicated
Concept introduction:In chair form all the
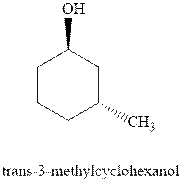
(c)
Interpretation: Most stable conformation for
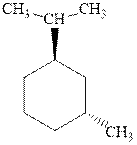
Concept introduction:In chair form all the
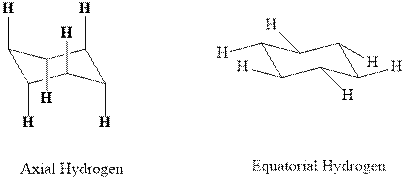
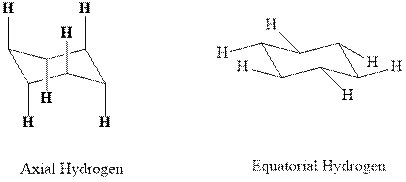
(d)
Interpretation: Most stable conformation for indicated
Concept introduction:In chair form all the
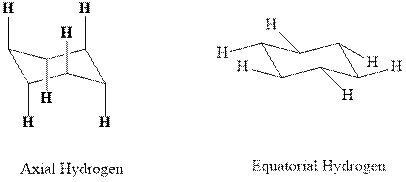
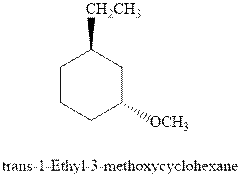
(d)
Interpretation: Most stable conformation for indicated
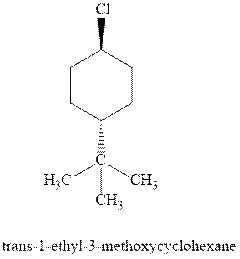
Concept introduction:In chair form all the
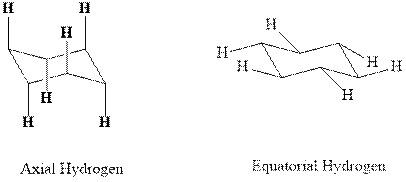
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: Structure and Function
- H C-Ć- C -CI Following is the structural formula of 1,2– dichloroethane. H H a) Draw Newman projections for all staggered and eclipsed conformations formed by rotation from 0° to 360° about the carbon- carbon single bond. b) Which staggered conformation(s) has the lowest energy; which has the highest energy? c) Which eclipsed conformation(s) has the lowest energy which has the highest energy?arrow_forward(a) Draw the most stable chair conformers of trans-1,3-diisopropylcyclohexane and trans-1,4-diisopropylcyclohexane. (b) Which is more stable: trans-1,3-diisopropylcyclohexane or trans-1,4-diisopropylcyclohexane?arrow_forward3)) Draw the most stable chair conformation of a 1,2,4-trimethylcyclohexane (Show all the hydrogens and indicate if the bond is axial or equatorial at the substitution).arrow_forward
- 4. (a) Draw a skeletal (line-bond) structure for 3,4-dimethylhexane. (b) Draw a sawhorse representation of any staggered conformation of this molecule looking down the carbon-3 to carbon-4 bond. (c) Draw a Newman projection looking down the carbon-3 to carbon-4 bond of the same conformation that you drew as a sawhorse representation.arrow_forwardcompound CH3 CH₂0 CH3 — OH C- CH3 1 CH3-N - CH3 CH₂ ||| -C-H 01C- | NH₂ CH3 family esterarrow_forward9) Draw trans-1,3-dichloro cyclohexane in the highest energy conformation.arrow_forward
- Draw the alternative chair conformations for the cis and trans isomers of 1,2-dimethylcyclohexane, 1,3- dimethylcyclohexane, and 1,4-dimethylcyclohexane. (a ) Indicate by a label whether each methyl group is axial or equatorial. (b) For which isomer(s) are the alternative chair conformations of equal stability? (c) For which isomer(s) is one chair conformation more stable than the other?arrow_forward(a) Draw and name all five isomers of formula C3H5F.(b) Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.arrow_forwardDraw each compound in its most stable conformation(s). Then draw it in its most symmetric conformation, and determine whether it is chiral.(a) 2-methylbutanearrow_forward
- Given each of the IUPAC names provided, draw the corresponding structure. (a) 2-cyclopropoxypentane;(b) 1,2-dimethoxy-4-propylcyclohexane; (c) 4-(1,1-dimethylethyl)-1,2-dipropoxycyclooctanearrow_forwardDraw (a) a Newman projection of the most stable conformation sighting down the C-3 C-4 bond and (b) a bond-line depiction of 2,2,5,5-tetramethylhexane.arrow_forwardFollowing is the Structural formal of Chloropropane H H H 2. H Draw Newman projections for all staggered and eclipsed conformation formed by rotation about C1 - C2 bond from 0° to 360°. b) Which Staggered conformation(s) has the lowest energy; which has the highest energy? c) Which eclipsed conformation(s) has the lowest energy, which has thhe highest energy?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





