
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 94P
Answer the following questions about alcohol B.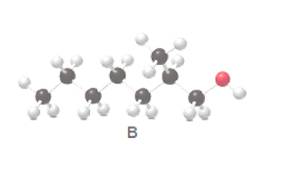
a. Give the IUPAC name.
b. Classify the alcohol as 1°, 2°, or 3°.
c. Draw the products formed when B is dehydrated with
d. What product is formed when B is oxidized with
e. Draw a constitutional isomer of B that contains an OH group.
f. Draw a constitutional isomer of B that contains an ether.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?
For the single step reaction: A + B → 2C + 25 kJ
If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state.
In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.
How many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?
Chapter 14 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 14.1 - Prob. 14.1PCh. 14.2 - Prob. 14.1PPCh. 14.2 - Classify each hydroxyl group in sorbitol as 1°,...Ch. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.3 - Prob. 14.2PPCh. 14.3 - Give the structure corresponding to each name a....Ch. 14.5 - Draw the products formed when each alcohol is...Ch. 14.5 - Prob. 14.6PCh. 14.5 - Prob. 14.7P
Ch. 14.5 - Prob. 14.4PPCh. 14.6 - Prob. 14.8PCh. 14.7 - Prob. 14.9PCh. 14.7 - Prob. 14.5PPCh. 14.7 - Prob. 14.10PCh. 14.7 - Name each ether. CH3OCH2CH2CH2CH3Ch. 14.7 - Prob. 14.11PCh. 14.8 - (a) Translate the hall and stick model of...Ch. 14.8 - Prob. 14.13PCh. 14.9 - Prob. 14.14PCh. 14.9 - Prob. 14.15PCh. 14.9 - Prob. 14.16PCh. 14.9 - Prob. 14.17PCh. 14.9 - Prob. 14.18PCh. 14.9 - Prob. 14.19PCh. 14.9 - Prob. 14.20PCh. 14.10 - Give the IUPAC name for each thiol.Ch. 14.10 - Prob. 14.21PCh. 14.10 - Prob. 14.22PCh. 14 - Classify each alcohol as 1°, 2°, or 3o a....Ch. 14 - Prob. 24PCh. 14 - Prob. 25PCh. 14 - Classify each halide hi A as 1°, 2°, or 3°. A is a...Ch. 14 - Prob. 27PCh. 14 - Draw the structure of a molecule that fits each...Ch. 14 - Draw the structure of the six constitutional...Ch. 14 - Draw the structure of the four constitutional...Ch. 14 - Draw the structure of l-propanethiol, a compound...Ch. 14 - Prob. 32PCh. 14 - Prob. 33PCh. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - Answer each question about alcohol B. Draw a...Ch. 14 - Prob. 37PCh. 14 - Give the IUAPC name for each alcohol.Ch. 14 - Prob. 39PCh. 14 - Prob. 40PCh. 14 - Prob. 41PCh. 14 - Prob. 42PCh. 14 - Draw the structures and give the IUPAC names for...Ch. 14 - Prob. 44PCh. 14 - Prob. 45PCh. 14 - Prob. 46PCh. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Which compound in each pair has the higher boiling...Ch. 14 - Rank the compounds in order of increasing melting...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Prob. 53PCh. 14 - Prob. 54PCh. 14 - Prob. 55PCh. 14 - Prob. 56PCh. 14 - Prob. 57PCh. 14 - Prob. 58PCh. 14 - Prob. 59PCh. 14 - Prob. 60PCh. 14 - Prob. 61PCh. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - Prob. 66PCh. 14 - Prob. 67PCh. 14 - Prob. 68PCh. 14 - Prob. 69PCh. 14 - Prob. 70PCh. 14 - Prob. 71PCh. 14 - Prob. 72PCh. 14 - Prob. 73PCh. 14 - Prob. 74PCh. 14 - Prob. 75PCh. 14 - Prob. 76PCh. 14 - Prob. 77PCh. 14 - Prob. 78PCh. 14 - Prob. 79PCh. 14 - Prob. 80PCh. 14 - With reference to the halogenated organic...Ch. 14 - Prob. 82PCh. 14 - Prob. 83PCh. 14 - Prob. 84PCh. 14 - Write out the chemical reaction that occurs when a...Ch. 14 - Prob. 86PCh. 14 - Prob. 87PCh. 14 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 14 - Prob. 89PCh. 14 - Prob. 90PCh. 14 - Prob. 91PCh. 14 - Prob. 92PCh. 14 - Prob. 93PCh. 14 - Answer the following questions about alcohol B....Ch. 14 - Prob. 95CPCh. 14 - Dehydration of alcohol C forms two products of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning 

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY