
(a)
Interpretation: The electron dot structure for the C element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
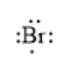
(a)
Explanation of Solution
The given element is C. It belongs to group 14. The
Here, the number of valence electrons will be 4. Thus, the electron dot structure can be written as follows:
(b)
Interpretation: The electron dot structure for Be element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
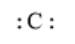
(b)
Explanation of Solution
The given element is Be. It belongs to group 2. The atomic number of Be atom is 4. The electronic configuration can be represented as follows:
Here, the number of valence electrons will be 2. Thus, the electron dot structure can be written as follows:
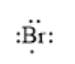
(c)
Interpretation: The electron dot structure for O element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
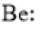
(c)
Explanation of Solution
The given element is O. It belongs to group 16. The atomic number of oxygen atom is 8. The electronic configuration can be represented as follows:
Here, the number of valence electrons will be 6. Thus, the electron dot structure can be written as follows:
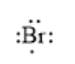
(d)
Interpretation: The electron dot structure for the F element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
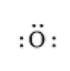
(d)
Explanation of Solution
The given element is F. It belongs to group 17. The atomic number of F atom is 9. The electronic configuration can be represented as follows:
Here, the number of valence electrons will be 7. Thus, the electron dot structure can be written as follows:
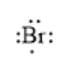
(e)
Interpretation: The electron dot structure for Na element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
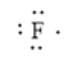
(e)
Explanation of Solution
The given element is Na. It belongs to group 1. The atomic number of sodium atom is 11. The electronic configuration can be represented as follows:
Here, the number of valence electrons will be 1. Thus, the electron dot structure can be written as follows:
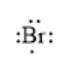
(f)
Interpretation: The electron dot structure for P element needs to be determined.
Concept Introduction: Electron dot structures of an atom of an element represent the number of electron/s present in its valance electron shell. In the electron dot structure, the symbol of an atom of an element is written and the number of valance electrons is arranged in pairs represented as dots around the symbol.
For example, the number of valance electrons in a bromine atom is 7; thus, its electron dot structure is represented as follows:
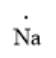
(f)
Explanation of Solution
The given element is P. It belongs to group 15. The atomic number of phosphorus atom is 15. The electronic configuration can be represented as follows:
Here, the number of valence electrons will be 5. Thus, the electron dot structure can be written as follows:
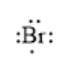
Chapter 7 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





