
Chemistry
4th Edition
ISBN: 9780078021527
Author: Julia Burdge
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25, Problem 82AP
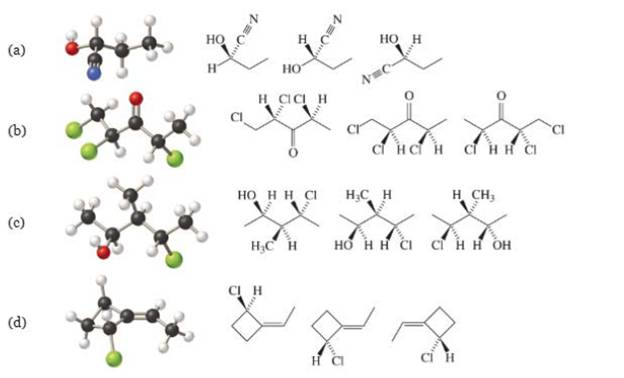
Match each molecular model with the correct line-wedge-dash structure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Determine a molecular formula, for example, CH4, from the line structure below.
(Specify elements in the following order: C, H, others (in alphabetical order). Example: C4H7CIOS)
Molecular formula
Determine a molecular formula, for example, CH4, from the line structure below.
(Specify elements in the following order: C, H, others (in alphabetical order). Example: C4H7CIOS)
HO.
Molecular formula
Select all the atoms that have at least one lone (unshared) pair of electrons.
• Gray = C; white H; red= 0; blue = N; dark green= Cl; brown Br; light green F; purple = 1; yellow=S; orange - P
Double click to select atoms.
You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens).
Convert the model below to a skeletal drawing.
wireframe
H
H
H
C
H
H
+ labels
H
Chapter 25 Solutions
Chemistry
Ch. 25.1 - Prob. 1PPACh. 25.1 - Prob. 1PPBCh. 25.1 - Prob. 1PPCCh. 25.2 - Prob. 1PPACh. 25.2 - Prob. 1PPBCh. 25.2 - Prob. 1PPCCh. 25.2 - Prob. 1CPCh. 25.2 - Prob. 2CPCh. 25.2 - Identify the name of the following compound: a)...Ch. 25.2 - Prob. 4CP
Ch. 25.2 - Prob. 5CPCh. 25.2 - Prob. 6CPCh. 25.3 - Prob. 1PPACh. 25.3 - Prob. 1PPBCh. 25.3 - Prob. 1PPCCh. 25.3 - Prob. 1CPCh. 25.3 - Prob. 2CPCh. 25.3 - Prob. 3CPCh. 25.3 - Prob. 4CPCh. 25.4 - Prob. 1PPACh. 25.4 - Prob. 1PPBCh. 25.4 - Prob. 1PPCCh. 25.5 - Prob. 1PPACh. 25.5 - Prob. 1PPBCh. 25.5 - Prob. 1PPCCh. 25.5 - Prob. 1CPCh. 25.5 - Prob. 2CPCh. 25 - Prob. 1QPCh. 25 - 25.2 Why was Wöhler’s synthesis of urea so...Ch. 25 - Prob. 3QPCh. 25 - Prob. 4QPCh. 25 - Prob. 5QPCh. 25 - Prob. 6QPCh. 25 - Prob. 7QPCh. 25 - Prob. 8QPCh. 25 - Prob. 9QPCh. 25 - Prob. 10QPCh. 25 - Prob. 11QPCh. 25 - Prob. 12QPCh. 25 - Prob. 13QPCh. 25 - Prob. 14QPCh. 25 - Prob. 15QPCh. 25 - Identify the functional groups in the...Ch. 25 - Prob. 17QPCh. 25 - Prob. 18QPCh. 25 - Prob. 19QPCh. 25 - Prob. 20QPCh. 25 - Prob. 21QPCh. 25 - Prob. 22QPCh. 25 - Prob. 23QPCh. 25 - Prob. 24QPCh. 25 - Prob. 25QPCh. 25 - Prob. 26QPCh. 25 - Prob. 27QPCh. 25 - Prob. 28QPCh. 25 - Prob. 29QPCh. 25 - Prob. 30QPCh. 25 - Prob. 31QPCh. 25 - Prob. 32QPCh. 25 - Prob. 33QPCh. 25 - Prob. 34QPCh. 25 - Prob. 35QPCh. 25 - Prob. 36QPCh. 25 - Prob. 37QPCh. 25 - Prob. 38QPCh. 25 - Prob. 39QPCh. 25 - Prob. 40QPCh. 25 - Prob. 41QPCh. 25 - Prob. 42QPCh. 25 - Prob. 43QPCh. 25 - Prob. 44QPCh. 25 - Prob. 45QPCh. 25 - Prob. 46QPCh. 25 - Prob. 47QPCh. 25 - Prob. 48QPCh. 25 - Prob. 49QPCh. 25 - Prob. 50QPCh. 25 - Prob. 51QPCh. 25 - Prob. 52QPCh. 25 - Prob. 53QPCh. 25 - Prob. 54QPCh. 25 - Prob. 55QPCh. 25 - Prob. 56QPCh. 25 - Prob. 57QPCh. 25 - Prob. 58QPCh. 25 - Prob. 59QPCh. 25 - Prob. 60QPCh. 25 - Prob. 61QPCh. 25 - Prob. 62QPCh. 25 - Prob. 63QPCh. 25 - Prob. 64QPCh. 25 - Prob. 65QPCh. 25 - Prob. 66QPCh. 25 - Prob. 67QPCh. 25 - Prob. 68QPCh. 25 - Prob. 69QPCh. 25 - Prob. 70QPCh. 25 - Prob. 71QPCh. 25 - Prob. 72QPCh. 25 - Prob. 73QPCh. 25 - Prob. 74QPCh. 25 - Prob. 75QPCh. 25 - Prob. 76QPCh. 25 - Prob. 77APCh. 25 - Prob. 78APCh. 25 - Prob. 79APCh. 25 - Prob. 80APCh. 25 - Prob. 81APCh. 25 - Match each molecular model with the correct...Ch. 25 - Prob. 83APCh. 25 - Prob. 84APCh. 25 - Prob. 85APCh. 25 - Prob. 86APCh. 25 - Prob. 87APCh. 25 - Prob. 88APCh. 25 - Prob. 89APCh. 25 - Prob. 90APCh. 25 - Prob. 91APCh. 25 - Prob. 92APCh. 25 - Prob. 93APCh. 25 - Prob. 94APCh. 25 - Prob. 95APCh. 25 - Prob. 96APCh. 25 - Prob. 97APCh. 25 - Prob. 98APCh. 25 - Prob. 99APCh. 25 - Prob. 100APCh. 25 - All alkanes give off heat when burned in air. Such...Ch. 25 - Prob. 102APCh. 25 - Prob. 1SEPPCh. 25 - Prob. 2SEPPCh. 25 - Prob. 3SEPPCh. 25 - Prob. 4SEPP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the given three-dimensional molecular structure, the differently colored spheres represent different types of atoms. Write a molecular formula for this molecule. molecular formula: C₁4H12 O Rotate X ❤ Rotate Y C ОН 3 Rotate Z Zoom In ПО Zoom Out A Label Atomsarrow_forwardDraw a Lewis structure for the molecule below, showing all lone pairs. You may abbreviate any methyl groups as CH₂. CHC1₂CH₂ CHCICH₂ SC Check -63 Q A N 2 563 W S Click and drag to start drawing a structure. X * 3 20 E D C 888 R F 8 50 V F5 T 1 G MacBook Air 6 B Move atoms, bonds, or curved arrows; move, co 7 H X N © 2024 McGraw Hill LLC. All Rights Re 8 E Ś DII FB K M F9 Carrow_forwardName the following organic compounds: compound CH3 1 CH₂ I CH₂ - CH₂ - CH CH₂ - CH3 CH3 | CH₂ 1 CH₂ CH₂ CH CH3 - CH3 CH3 If - CH₂-C - CH₂ CH₂ CH3 CH₂ - CH₂ - name 0 0 Xarrow_forward
- 2. Infer In a line-angle formula, each line repre- sents a carbon-carbon bond. Each end of a line, as well as the intersection of lines, represents a carbon atom. Knowing that carbon always forms four covalent bonds, use labeled drawings to summarize how to determine the number of hydrogen atoms bonded to each carbon in a line-angle formula.arrow_forwardWrite the name of the hydrocarbon molecules below. CH3 CH3 CH2 ĆH .CH2 .CH2 CH3 CH2 CH ČH3arrow_forwardFor the molecule below, determine the polarity of the polar bonds. Use 5+ and 6- on individual atoms AND crossed arrows for bond polarity to indicate your answer. H O H-O CH3arrow_forward
- Shown below is the skeletal structure of an organic compound. What is its molecular formula? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. b с d C70 C5H5O С6H₂O C6H100 e C5H110arrow_forwardProvide a reasonable Lewis structure below each molecular formula. Please note that you must indicate the proper number of lone pairs CH3CH2OCH3 (CH3)3NH Na OCH(CH3)2 AlCl3 H3CCH(OH)CH2CO2H H3CCNOarrow_forwardHow many of the following compounds are molecular? Br2 CaF2 BaSO4 SrF2 NH3 HNO2arrow_forward
- Convert each of the following molecular models into a skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray=C, red =O, blue =N, ivory = H)arrow_forwardConvert each of the following molecular models into a skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray = C, red = O, blue = N, ivory = H).arrow_forwardDetermine the compound (name or structure) from the data. Explain features from each data. Molecular formula: C6H5Br .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY