
Interpretation: To show the reaction when glycine acts as a base in reaction with water.
Concept introduction: Amino acids are amphoteric in nature since it has both carboxyl and amino acid groups which means they can behave as both acid and base.
Answer to Problem 9STP
The chemical equation where glycine acts as a base can be depicted as follows:
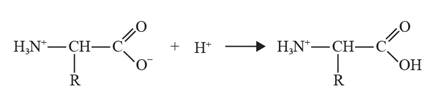
Explanation of Solution
Glycine can act as both acids as well bases. About all amino acids exist in zwitter ions. In acidic conditions, glycine can act as a base.
The reaction of glycine with acid leads to the formation of a positive ion as the carboxylate ion gains a proton.
The chemical equation can be depicted as follows:
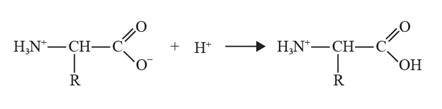
In acidic conditions, glycine behaves as a base, it leads to the formation of a positive ion as the carboxylate ion gains a proton.
Chapter 24 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Please correct answer and don't use hand ratingarrow_forwardPick 1 sets of two molecules from the list in the picture and provide a simulation of 2D NMR (H-H COSY and HSQC). I dont fully understand how to do it, so I would like to see how you do itarrow_forwardPlease correct answer and don't use hand ratingarrow_forward
- Use the molecular orbital diagram shown to determine which of the following are paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2P 2P 02p 015 B, C, Narrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





