
(a)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with
(a)
Answer to Problem 128A
The structural formula for heptane is represented as follows:
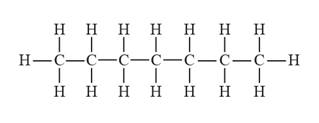
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In heptane, there is a long continuous carbon chain containing seven carbon atoms. Thus, the structural formula for heptane is given as follows:
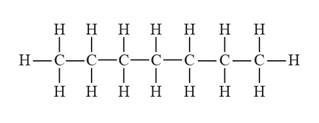
(b)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(b)
Answer to Problem 128A
The structural formula for 2-phenylbutane is represented as follows:
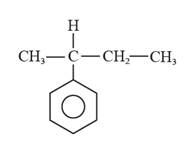
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 2-phenylbutane, there is a phenyl ring i.e., a parent ring, and butane is substituted at the second carbon on this phenyl ring. Thus, the structural formula for 2-phenylbutane is given as follows:
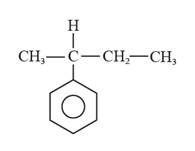
(c)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(c)
Answer to Problem 128A
The structural formula for 2-methyl-3-hexene is represented as follows:
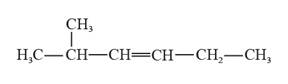
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 2-methyl-3-hexene, the longest continuous chain is six carbon atoms chain that has a double bond at the third carbon. There is a methyl substituent that is present on the second carbon in this long continuous chain. Thus, the structural formula for 2-methyl-3-hexene is given as follows:
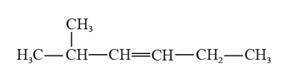
(d)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(d)
Answer to Problem 128A
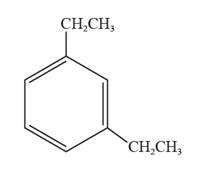
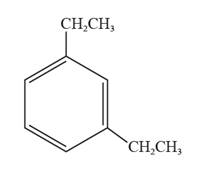
The structural formula for 1,3-diethylbenzene is represented as follows:
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 1,3-diethylbenzene, the parent ring is benzene, and ethyl groups are substituted on the first and the third carbon atoms. Thus, the structural formula for 1,3-diethylbenzene is given as follows:
Chapter 24 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





