
(a)
Interpretation: To write the IUPAC name for the given compound.
Concept Introduction: In order to give names to organic compounds,
(a)
Answer to Problem 130A
The IUPAC name for the given compound is cyclopentane.
Explanation of Solution
The given compound is cyclic and consists of five carbon atoms that are bonded with two hydrogen atoms.
In IUPAC nomenclature for naming cyclic compounds depends on the longest chain of carbons that are connected via single bonds. The prefix to be used while writing cyclic compounds is “cyclo” followed by the name of the continuous chain of carbon atoms.
The given compound is represented as follows:
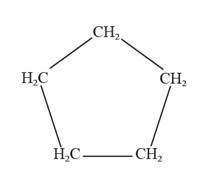
So, the given compound has a continuous cyclic ring containing five carbon atoms (i.e., pentane). Thus, the IUPAC name for the compound is cyclopentane.
(b)
Interpretation: To write the IUPAC name for the given compound.
Concept Introduction: In order to give names to organic compounds, IUPAC nomenclature is used.
(b)
Answer to Problem 130A
The IUPAC name for the given compound is 2-methyl-2-propanol.
Explanation of Solution
The rules for writing IUPAC names for the organic compounds are as follows:
- The root word indicates the longest continuous chain in the compound which is also known as the parent chain.
- Then suffixes and prefixes must be written as per the substituents, side chains, or
functional groups .
The given compound is represented as follows:
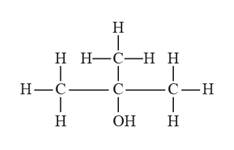
So, for the given compound, the longest possible chain is three carbon atom chain (i.e., propane), there is a substituent that is present at the second carbon which also contains an alcohol functional group ( for alcohols, the suffix to be used is “ol”).
Therefore, the IUPAC name for the given compound is 2-methyl-2-propanol.
(c)
Interpretation: To write the IUPAC name for the given compound.
Concept Introduction: In order to give names to organic compounds, IUPAC nomenclature is used.
(c)
Answer to Problem 130A
The IUPAC name for the given compound is 3-pentanone.
Explanation of Solution
The rules for writing IUPAC names for the organic compounds are as follows:
- The root word indicates the longest continuous chain in the compound which is also known as the parent chain.
- Then suffixes and prefixes must be written as per the substituents, side chains, or functional groups.
The given compound is represented as follows:
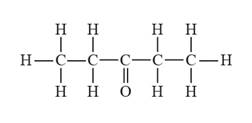
So, for the given compound the longest possible continuous carbon chain is five carbon atoms (i.e., pentane) and it has a ketone functional group that is present on the third carbon. For
Therefore, the IUPAC name for the given compound is 3-pentanone.
Chapter 24 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





