
Interpretation: To show the reaction when glycine acts as an acid in reaction with water.
Concept introduction: Amino acids are amphoteric in nature since it has both carboxyl and amino acid groups which means they can behave as both acid and base.
Answer to Problem 8STP
The chemical equation where glycine acts as an acid can be depicted as follows:
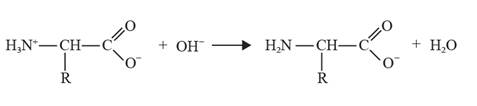
Explanation of Solution
Glycine can act as both acids as well bases. In strongly alkaline conditions, glycine can act as a base. About all amino acids exist in zwitter ions.
The reaction of glycine with a base leads to the formation of a negative ion when an ammonium ion loses a proton.
The chemical equation can be depicted as follows:
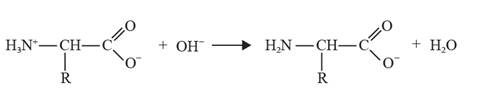
In alkaline conditions, glycine behaves as an acid, it leads to the formation of a negative ion when an ammonium ion loses a proton.
Chapter 24 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Would this align with acetone 2 ว 10 20 STUDENT LAB NOTE 26 3133 30 42 41 44 96 56 60 40 50 50 8-8-8 70 70 74 7375 80 60 90arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardPick 1 sets of two molecules from the list in the picture and provide a simulation of 2D NMR (H-H COSY and HSQC). I dont fully understand how to do it, so I would like to see how you do itarrow_forward
- Please correct answer and don't use hand ratingarrow_forwardUse the molecular orbital diagram shown to determine which of the following are paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2P 2P 02p 015 B, C, Narrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





